

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
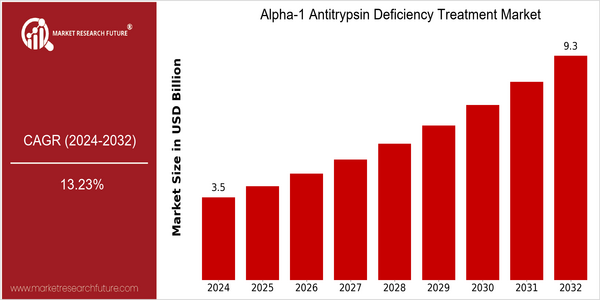
Alpha 1 Antitrypsin Deficiency Treatment Market Size Snapshot
| Year | Value |
|---|---|
| 2024 | USD 3.52 Billion |
| 2032 | USD 9.28 Billion |
| CAGR (2024-2032) | 13.23 % |
Note – Market size depicts the revenue generated over the financial year
The alpha-1 antitrypsin deficiency treatment market is projected to reach $ 3,523.1 million in 2024 and is expected to reach $ 9,288.6 million by 2032. This strong growth reflects a CAGR of 13.23% over the forecast period. The growing prevalence of AATD, together with an increase in awareness and improved diagnostics, is driving the demand for effective treatment options. Also, the development of health systems and the increasing emphasis on precision medicine and targeted therapies are promoting the market. The development of novel therapies such as gene therapy and monoclonal antibodies are also contributing to this growth. Grifols, CSL Behring and Kamada are the leading companies in this field, and they are investing in research and development, establishing strategic alliances and launching new products to increase their market share. Grifols has been expanding its portfolio of treatments for AATD, while CSL Behring has been expanding its distribution network to improve patient access. These strategic moves show the market is highly dynamic and that AATD treatments are set to continue to grow.
Regional Deep Dive
The alpha-1-antitrypsin deficiency treatment market is characterized by a growing awareness of the disease and the development of therapeutic options in various regions. In North America, the market is driven by a strong health care system, a high prevalence of the disease and a strong emphasis on research and development. In Europe, the regulatory framework is diverse and access to treatment varies, while in Asia-Pacific, a rising number of diagnoses and an increase in treatment availability, despite access issues, is a major driver of the market. The Middle East and Africa are characterized by a lack of awareness and resources, while Latin America is gradually improving its treatment landscape through collaborations and government initiatives.
North America
- Recent approvals by the FDA for the treatment of alpha-1-antitrypsin deficiency have expanded the treatment options for patients and have increased market growth.
- The Alpha Foundation is an organization that actively promotes public education and awareness of the disease. Consequently, the rate of diagnosis and treatment should increase.
- The presence of large pharmaceutical companies like Grifols and CSL Behring has facilitated innovation and competition, which have benefited patients.
Europe
- The European Medicines Agency (EMA) has shortened the approval procedure for new medicines, which will encourage pharmaceutical companies to invest in research and development of new therapies for Alpha-1 Antitrypsin Deficiency.
- Germany and France are introducing national health programmes that are intended to improve access to care.
- The market is influenced by the reimbursement policies of the various countries of Europe, which may affect the access to therapy and the market.
Asia-Pacific
- In countries such as Japan and Australia, where the number of alpha-antitrypsin deficiency patients has increased considerably, the demand for treatment has also increased.
- Moreover, there are now emerging in the developing world some creative new arrangements between local health services and international pharmaceutical companies to improve access to medicines.
- In the meantime, however, the development of the market is hampered by regulatory difficulties and the poor medical system in some countries. But the efforts made to improve access to medical care are encouraging.
MEA
- In the Middle East and Africa, the limited awareness of alpha-1 antitrypsin deficiency is a significant barrier to the diagnosis and treatment of this disease. This has prompted local health authorities to take action.
- Governments and non-governmental organizations are collaborating to improve the screening of children and access to treatment in underserved areas.
- The diversity of the health systems and the economic disparities create unique challenges for the penetration of the markets and patient access to therapies.
Latin America
- In Brazil and Mexico, a national health policy is being developed for rare diseases, including alpha-1 antitrypsin deficiency, which is expected to improve access to care.
- The number of patients and the need for effective treatments have increased the interest of the pharmaceutical companies in the region.
- Local governments and international organizations have collaborated to raise awareness and improve education and diagnosis.
Did You Know?
“In the Caucasian population, approximately 1 in 2,500 people suffer from alpha-antitrypsin deficiency, making it one of the most common hereditary disorders in this population.” — Alpha-1 Foundation
Segmental Market Size
Alpha-1 Antitrypsin Deficiency (AATD) is a growing part of the broader respiratory and genetic disorders treatment landscape. It is currently experiencing increased demand due to an increased awareness of the condition and its associated health risks, especially in the high-prevalence regions of Europe and North America. Also driving growth is the increase in genetic testing and personal medicine, which has led to more patients being diagnosed and seeking effective treatments.
In the field of biotechnology, a number of factors are pushing the market, such as the development of new treatments such as augmentation therapy and gene therapy. Regulations and the possibility of faster approval of rare diseases, are also pushing the market for new treatments. Adoption is currently transitioning to a more mature stage, with Grifols and Kamada leading the development and distribution of treatments for rare diseases. The main application is the treatment of AATD and the liver and lung diseases that accompany it, and the main use is in specialized hospitals and clinics. The macro-economic trend of increasing medical expenses and the focus on rare diseases are pushing the market. The recombinant DNA and monoclonal antibody technology will shape the future of AATD treatment.
Future Outlook
From 2024 to 2032, the global alpha-antitrypsin deficiency treatment market is expected to grow at a CAGR of 13.23%. The main reasons for this are the increasing prevalence of alpha-antitrypsin deficiency (AATD), the growing awareness of health care professionals, and the advancements in the treatment modalities. As the world's population ages and the prevalence of chronic respiratory diseases increases, the demand for effective therapies is expected to increase, which in turn will lead to a higher penetration of the market and the penetration of the treatment among patients with AATD, which could reach more than 50% in 2032.
Despite the difficulties of a new therapy for AATD, the outlook for the future is bright, and the advent of novel biologicals and gene therapies may revolutionize the treatment of AATD. Also, research on the development of a personalized medicine and targeted therapies will probably increase the effectiveness of therapy and the outcome of the patient. Also, the supportive legislation and increased funding for rare diseases will probably facilitate the market expansion. Telemedicine and digital health solutions will also play an important role in increasing access to treatment and monitoring the progress of the disease, further increasing the growth of the market. In general, the AATD treatment market will grow and develop a lot in the coming years, primarily driven by innovation and a commitment to improving patient care.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 13.23% |
Alpha 1 Antitrypsin Deficiency Treatment Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









