

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Anti Nuclear Antibody Testing Market Size Snapshot
| Year | Value |
|---|---|
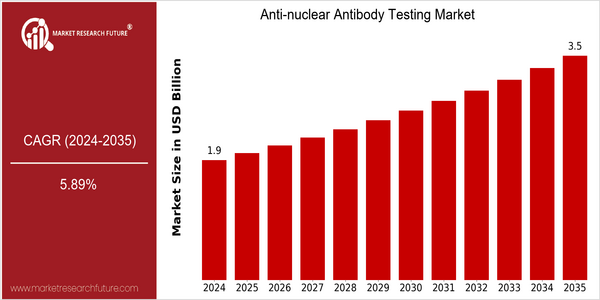
| 2024 | USD 1.86 Billion |
| 2035 | USD 3.5 Billion |
| CAGR (2025-2034) | 5.89 % |
Note – Market size depicts the revenue generated over the financial year
Anti-nuclear antibody testing is a market with significant growth potential, with a current market size of $1.8 billion in 2024, projected to reach $3.5 billion by 2035. This translates into a CAGR of 5.89 % from 2025 to 2035. The increasing prevalence of autoimmune diseases, coupled with the advances in diagnostics, has accelerated the demand for ANA testing. The market is expected to grow with the advent of newer and more accurate testing methods and the integration of artificial intelligence in laboratory processes. Thermo Fisher Scientific, Siemens, and Abbott Laboratories are the leading companies in the ANA testing market. Strategic alliances and collaborations aimed at developing next-generation ANA testing solutions are also expected to boost the market. Recent product launches with higher sensitivity and screening capabilities are expected to propel the market in the coming years.
Regional Deep Dive
The ANA market is characterized by considerable regional variations, driven by factors such as disease prevalence, regulatory frameworks, and healthcare infrastructure. In North America, the market is supported by the presence of a strong healthcare system and a high prevalence of autoimmune diseases. In Europe, the market is backed by the stringent regulatory frameworks that ensure high-quality testing. The Asia-Pacific region is expected to grow rapidly, owing to the rising disease awareness and increasing healthcare expenditure. The Middle East and Africa region, however, is expected to face challenges, such as lack of access to testing and lack of awareness. Latin America is gradually gaining traction, owing to the rising healthcare expenditure and the growing focus on chronic disease management.
North America
- The Food and Drug Administration of the United States has recently approved several new ANA tests which are said to improve the accuracy and speed of diagnosis and are expected to lead to a sharp rise in the number of ANA tests in the United States.
- Several major companies, including Thermo Fisher Scientific and Siemens Healthineers, are investing in the development of new diagnostic systems that are expected to improve laboratory processes and patient care.
- Moreover, the growing trend of telemedicine in North America facilitates consultations and follow-ups, thus making the ANA test more accessible to patients in remote areas.
Europe
- The European Medicines Agency has issued new guidelines for the standardization of ANA testing. The ANA test is expected to become more reliable and uniform in all member states.
- At present, such companies as Euroimmun and Roche Diagnostics are leading the field in the development of such serological tests, which can detect several autoimmune diseases at the same time. The demand for such a comprehensive test is growing.
- The social and cultural factors, the strong emphasis on preventive medicine in countries such as France and Germany, are leading to the ANA test being included in the normal health check.
Asia-Pacific
- China and India are experiencing an increase in ANA tests, resulting from the rising prevalence of autoimmune diseases and the increasing availability of medical resources. As a result, domestic companies like Wondefo Biotech have been able to develop their ANA tests.
- The World Health Organization (WHO) has initiated a program of strengthening laboratory capacity in developing countries. This is expected to increase access to ANA testing in underdeveloped areas.
- In the sphere of diagnostics, rapid tests are gaining ground, especially in the rural areas where access to conventional laboratories is restricted.
MEA
- In Africa, the African Society of Medical Laboratories is striving to improve the standard of laboratories and the access to ANA testing in different countries, in order to meet the gap in the diagnosis of autoimmune diseases.
- In the Middle East, the increased awareness of rheumatic diseases promoted by the Saudi Society for Rheumatology is expected to increase the number of cases of rheumatic diseases.
- ANA testing is available in all countries of the world, but in some countries, such as those in Africa, it is not available, and this requires new solutions to ensure access to it.
Latin America
- Brazil and Mexico have been able to develop their medical services, and these have improved access to ANA testing and thereby enhanced the diagnostic capabilities.
- The local industry is in collaboration with international firms in order to increase the availability of advanced ANA testing kits to meet the growing demand for a precise diagnosis of autoimmune disorders.
- The trend towards prevention and chronic disease management is influencing public health policy and has increased the budget for autoimmune diseases.
Did You Know?
“It is known that about 50% of lupus patients test positive for an antinuclear antibody, and thus this test is a crucial diagnostic tool for this autoimmune disease.” — American College of Rheumatology
Segmental Market Size
Anti-nuclear antibodies are a vital part of the diagnostics market and are currently experiencing steady growth. This market is important for the diagnosis of autoimmune diseases, which are becoming more common and more widely recognized. In this field, the main growth drivers are the increasing number of autoimmune diseases, technological progress, the growing importance of early detection and the trend towards individualized medicine.
The use of ANAs is now in a stage of maturity, and Thermo Fisher and the European Syndicate of Immunology have taken the lead. The companies have developed a robust testing platform that improves the accuracy and speed of ANAs. The main applications are in the diagnosis of lupus and rheumatoid arthritis, and the main contact points are in hospitals and laboratories. The use of ANAs is expected to grow at a CAGR of 6.7% from 2018 to 2023, accelerated by the rising trend of precision medicine and the integration of artificial intelligence into diagnostics. Also, the support of the government for new methods and the trend of decentralized medical care will lead to a further development of the industry.
Future Outlook
Anti-nuclear Antibodies (ANA) Testing Market is expected to grow at a significant CAGR of 5.89 % during the forecast period of 2024 to 2035. This growth is mainly due to the rising prevalence of autoimmune disorders, which are increasingly being diagnosed by ANA testing. The awareness of these disorders is increasing, which is expected to increase the uptake of ANA testing in clinical and laboratory settings. In 2035, the percentage of patients with autoimmune symptoms who will undergo ANA testing is expected to be approximately 30 %, a sign of growing reliance on this diagnostic tool.
The main technological developments, such as the development of more sensitive and specific diagnostic methods, are expected to increase the market's growth. In the near future, developments in multiplexing and automation of laboratories will simplify the diagnostic process, reduce the time to results and improve the quality of the results. Also, a favorable policy environment and the increased budget for autoimmune diseases will contribute to the expansion of the market. The emergence of artificial intelligence in diagnostics and the development of a new direction in medicine will also have a significant impact on the ANA diagnostics market. The market will continue to develop and provide new opportunities for all market participants and new opportunities for diagnostics for health care professionals.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 11.3% |
Anti Nuclear Antibody Testing Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









