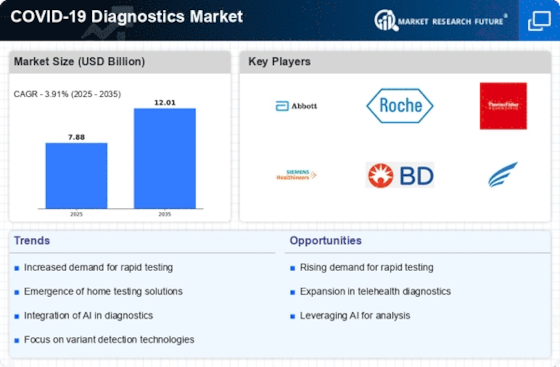
Top Industry Leaders in the COVID 19 Diagnostics Market
 *Disclaimer: List of key companies in no particular orderLatest COVID-19 diagnostics Companies Update
*Disclaimer: List of key companies in no particular orderLatest COVID-19 diagnostics Companies Update
-
February 2023: The SARS-CoV-2 Total Antibody Test was just released by NanoSpot.ai in the European market. The agglutination-based technology and a smartphone app with artificial intelligence (AI) are used to carry out the semi-quantitative antibody test at the point of care. The item was certified by the company in May, enabling its use in the European Union and other countries that recognize the CE Mark. The whole process, from sample collection to result acquisition, is finished in less than three minutes and does not require the use of a sophisticated diagnostic tool. The test has shown to be 100% specific and 97.6% sensitive, making it appropriate for usage in both personal and professional situations. -
April 2023: The first 37 real-time PCR test kits with the CE-IVD certification were released by Thermo Fisher Scientific in April 2023. The aforementioned kits are intended for use with the QuantStudio Dx equipment range that the company sells. They are anticipated to be made available to customers at a later time this year. At the European Congress of Clinical Microbiology and Infectious Diseases, Thermo Fisher displayed test kits made for screening human papillomavirus and herpes simplex virus using their QuantStudio 5 Dx (QS5 Dx) real-time PCR technology. During the first half of 2023, Thermo Fisher plans to roll out more diagnostic tests for sexually transmitted and blood-borne disorders like chlamydia, gonorrhea, and HIV. The business then intends to introduce tests for hepatitis, infections in transplant recipients and immunocompromised people, and respiratory tract infections.
List of COVID-19 diagnostics Key companies in the market
- Becton, Dickinson & Company (US)
- Bio-Rad Laboratories (US)
- Danaher Corporation (US)
- Abbott (US)
- Hoffmann-La Roche Ltd (Switzerland)
- Biomerieux SA (France)
- Genmark Diagnostics (US)
- Thermo Fisher Scientific (US)
- Qiagen (Germany)
- Siemens AG (Germany)