Top Industry Leaders in the Europe Sterility Testing Market
 Latest Sterility testing Companies Update
Latest Sterility testing Companies Update
July 2023: Ten23 Health recently introduced quality control (QC) services in Switzerland, which are designed to conduct stability and release testing of sterile commercial and clinical drug products in accordance with international cGMP standards. Two locations of ten23 will house the new quality control division in order to accommodate a variety of testing needs and to ensure that each location is in optimal harmony with our other offerings. In Basel ten23 Health, physico-chemical testing, including identity, purity, and content, will be provided in accordance with pharmacopeial standards to assure the stability and release of sterile pharmaceutical drug products intended for clinical and commercial use. Ten23 will incorporate these services with the pharmaceutical development service it offers in Basel. The pharmaceutical industry's partner headquartered in Basel has established a new quality control (QC) division to assist in the release and stability testing of sterile drug products for commercial and clinical use, in accordance with international cGMP standards.
November 2023: MilliporeSigma, a life science enterprise of Merck KGaA, Darmstadt, operating in the United States and Canada, introduced ChemisTwinTM, the first digital reference materials platform capable of employing calibrated algorithm-based digital references for automated analysis of sample purity, identification, and degradation of compounds. By utilizing digital signatures for over 1,500 reference materials, this automation and calibration instrument aids scientists in guaranteeing the safety and quality of pharmaceuticals throughout the entire research and development process, including quality control and assurance testing. Life sciences are being disrupted by the expanding use of digital technologies that are supported by high-quality data. The ability to digitize reference materials represents an enormous advance in the protection of consumer goods and medications around the globe. The user-friendly and intuitive nature of ChemisTwin™ facilitates the progression of scientific inquiry, product innovation, quality assurance, and adherence to regulatory standards.
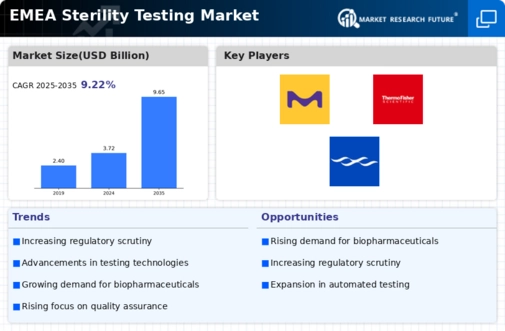
List of Sterility testing Key companies in the market
- Merck KGaA (Germany)
- bioMérieux SA (France)
- SGS SA (Switzerland)
- Sartorius AG (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Toxikon, Inc. (U.S.)
- Charles River (U.S.)