

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
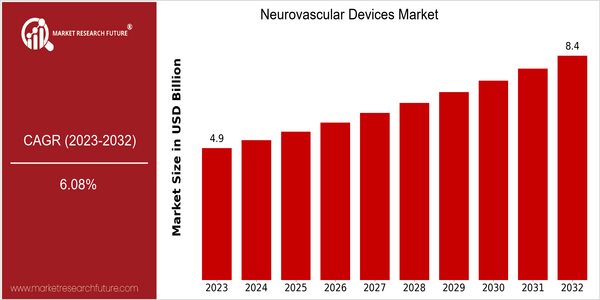
Neurovascular Devices Market Size Snapshot
| Year | Value |
|---|---|
| 2023 | USD 4.94 Billion |
| 2032 | USD 8.4 Billion |
| CAGR (2024-2032) | 6.08 % |
Note – Market size depicts the revenue generated over the financial year
The neuro-vesical devices market is estimated to be worth around $ 4.94 billion in 2023, and is expected to reach $ 8.4 billion by 2032, at a robust CAGR of 6.08 % from 2024 to 2032. The growth in the demand for neuro-vesical devices is due to the increasing prevalence of neural disorders, the development of medical technology and the aging of the population, which makes it more susceptible to conditions such as stroke and aneurysms. The neuro-vesical devices market is also driven by the increasing use of minimally invasive surgical procedures, which have a positive effect on the patient's recovery time and reduce hospital stays. Also, the development of advanced imaging methods and the integration of artificial intelligence in the diagnostics and treatment planning are expected to drive the growth of the market. Medtronic, Stryker and Penumbra are the leading companies in the neuro-vesical devices market. These companies are constantly launching new products and establishing strategic alliances to increase their market presence and strengthen their product portfolio. Recent collaborations to develop the next generation of neuro-vesical stents and thrombectomy devices demonstrate the industry's commitment to improving patient outcomes and driving market growth.
Regional Deep Dive
Neurovascular devices market is experiencing a significant growth in various regions, owing to the increasing prevalence of neuro-vascular diseases, technological advancements, and a growing geriatric population. In North America, the market is characterized by a high healthcare expenditure, extensive research and development, and a strong presence of key players. In Europe, the market is characterized by a diverse regulatory framework and a focus on novel treatment options, while in the Asia-Pacific region, the market is experiencing a rapid adoption of neuro-vascular devices, owing to the improving healthcare facilities and increasing awareness. Middle East and Africa (MEA) are characterized by limited access to advanced medical technology, which is gradually improving with the support of the government. Latin America is also an emerging market with increasing healthcare expenditure and rising demand for neuro-vascular procedures.
North America
- Recent approvals of a number of new neuro-vascular devices, such as stent-retrievers and flow-diverters, have further increased the therapeutic options for treating aneurysms and ischemic strokes.
- Medtronic and Stryker are investing heavily in R & D, focusing on minimally invasive surgical techniques to reduce the recovery time and improve the patient's outcome.
- In view of the increasing occurrence of cerebral haemorrhage and other vascular disorders, and the increasing number of the elderly population, a demand for improved neurovascular surgery is expected.
Europe
- European Union regulations aimed at improving the safety and efficacy of medical devices have affected the development and approval of neuro-vascular devices.
- A new generation of companies such as Penumbra and Siemens Healthineers is introducing new and more effective neuroimaging and treatment methods.
- The cultural context of the different European countries, a strong emphasis on patient-centred care and the integration of digital health solutions, shapes the market dynamics.
Asia-Pacific
- Japan and China are rapidly adopting neurovascular devices, as they have made significant investments in their medical care systems and the public is becoming more aware of neurovascular diseases.
- Local manufacturers such as the MicroPort Scientific Company have stepped in to meet the region’s needs.
- The emergence of neuro-vascular devices in emerging markets is driven by the growing government initiatives for the development of health care and the increasing availability of health care.
MEA
- There is an increase in the collaboration between the government and the private sector to improve access to care, which is essential for the introduction of neurovascular devices.
- A number of organizations, such as the World Health Organization, have taken the initiative to raise awareness of the dangers of stroke and the need for treatment and prevention.
- The economies of the countries of the Middle East and Africa, as well as the availability of advanced medical technology, are affecting the market in different ways.
Latin America
- Foreign investment in health care is growing in Latin America. Companies like Boston Scientific are expanding their operations there.
- Brazil and Mexico have shortened the approval time for medical devices, thus facilitating the entry of new neurovascular solutions into the market.
- A growing awareness of the importance of early intervention in the treatment of neuro-vascular disorders is driving the demand for neuro-vascular devices in the region.
Did You Know?
“Stroke is caused by occlusion of the blood vessel, and approximately 87% of all strokes are occlusions, which can be effectively treated with neurovascular devices such as stent-retrievers and thrombectomy devices.” — American Stroke Association
Segmental Market Size
Neuro-Vascular Devices Market is a dynamic market in the broader medical device industry, which is experiencing robust growth driven by increasing rates of stroke and aneurysms. The neuro-vascular devices market is a critical contributor to the advancement of patient outcomes through the development of stents and embolization devices. The demand for these devices is being driven by a growing aging population, which is more susceptible to neuro-vascular conditions, and the advancement of minimally invasive surgical techniques, which have shortened the time for patient recovery and reduced the length of hospital stays.
The neuro-vascular devices market is currently in a phase of maturity. The main manufacturers, Medtronic and Stryker, are mainly active in the innovation and penetration of the market, particularly in North America and Europe. The most important applications are the treatment of ischemic strokes and cerebral aneurysms, where devices such as thrombectomy systems and flow diverters are used. The development of telemedicine and remote patient monitoring, which was accelerated by the CovD pandemic, is a growth driver in this market. Artificial intelligence and machine learning are also having an effect on the development of neuro-vascular devices, with a view to improving diagnostic accuracy and treatment success rates.
Future Outlook
The neuro-vasculature devices market is expected to grow at a CAGR of 6.08% from 2023 to 2032. The growth is mainly due to the rising prevalence of neuro-vasculature disorders such as stroke and aneurysms, in conjunction with the aging population, which is more susceptible to these conditions. As early diagnosis and intervention are becoming increasingly important in the healthcare system, the demand for advanced neuro-vasculature devices is expected to increase, leading to a higher penetration of these devices in both developed and emerging markets.
A great number of technical improvements, such as the development of minimally invasive surgical methods and the introduction of new diagnostic tools, are destined to revolutionize the neuro-vascular field. The use of artificial intelligence and machine learning in the development of diagnostic tools is expected to improve the accuracy and efficiency of neuro-vascular procedures, and to further drive market growth. The government’s support for medical research is also expected to encourage innovation and accelerate the introduction of new products. By 2032, it is expected that the use of neuro-vascular devices will have reached an unprecedented level, with a large number of people benefiting from these life-saving tools.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 5.7% (2022-2030 Base Year 2021 Forecast Period 2022-2030 Historical Data 2020 Forecast Units Value (USD Billion) Report Coverage Revenue Forecast, Competitive Landscape, Growth Factors, and Trends Segments Covered Product, Therapeutic Application, End User Geographies Covered North America, Europe, Asia-Pacific, and Rest of the World (RoW) Key Vendors Stryker, Medtronic, Johnson & Johnson Services Inc., TERUMO CORPORATION, Abbott, Merit Medical Systems Inc., Medikit Co. Ltd., Penumbra Inc., MicroPort Scientific Corporation, Evasc, Rapid Medical, Neuravi, L. Gore & Associates Inc., OxfordEndovascular, Sensome, Blockade Medical LLC., Delaware Corporation, Secant Group LLC, Gynesonics Key Market Opportunities Massive Demand for Minimally Invasive Procedures to Present Opportunities Key Market Drivers Expanding Patient Pool Fosters Demand for Neurovascular Devices |
Neurovascular Devices Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









