

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Market Size Snapshot
| Year | Value |
|---|---|
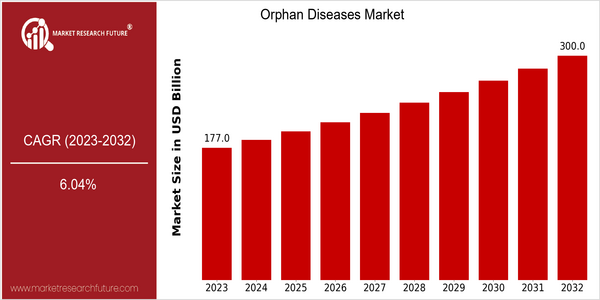
| 2023 | USD 177.01 Billion |
| 2032 | USD 300.0 Billion |
| CAGR (2024-2032) | 6.04 % |
Note – Market size depicts the revenue generated over the financial year
The orphan drug market is valued at $177.01 billion in 2023, and is projected to reach $300 billion by 2032, growing at a CAGR of 6.0% from 2024 to 2032. This growth trajectory is due to the growing awareness and recognition of rare diseases in the health sector, which is largely due to the advancements in biotechnology, government incentives, and the growing patient advocacy movement. The increasing focus on developing targeted therapies and personalized medicines is bringing new opportunities and investment in the orphan drug market. The rising prevalence of rare diseases is driving the orphan drug market growth. Gene therapy and CRISPR are transforming the treatment of rare diseases by enabling more effective and tailored solutions. The major players in the orphan drug market, such as Vertex, Amgen, and Novartis, are adopting strategic initiatives, such as collaborations and strategic alliances, to enhance their product portfolios and expand their market presence. These collaborations have shown the industry’s commitment to addressing the unmet medical needs and improving the patient outcomes.
Regional Market Size
Regional Deep Dive
The orphan drug market is characterised by the increasing importance of rare diseases that only affect a small proportion of the population, but which in aggregate affect millions. The market is characterised by the increasing awareness of these diseases, the development of new biotechnological treatments and the supportive regulatory frameworks in the five regions of North America, Europe, Asia-Pacific, the Middle East and Africa and Latin America. Each region has its own characteristics based on the health care system, the economic situation and the cultural attitude towards rare diseases. This creates different opportunities for the pharmaceutical and biotechnology industry.
Europe
- The European Medicines Agency (EMA) has implemented a centralized procedure for orphan drug designation, streamlining the approval process and encouraging pharmaceutical companies to invest in rare disease research.
- Notable collaborations, such as the partnership between Novartis and the European Organization for Rare Diseases (EURORDIS), are enhancing patient access to innovative therapies and fostering a more robust ecosystem for orphan drug development.
Asia Pacific
- Countries like Japan and South Korea are increasingly recognizing the importance of orphan drugs, with Japan's Pharmaceuticals and Medical Devices Agency (PMDA) offering expedited review processes for orphan drug applications.
- Emerging biotech firms in China are focusing on orphan diseases, supported by government initiatives aimed at boosting innovation in the healthcare sector, which is expected to lead to a surge in new therapies.
Latin America
- Brazil has introduced regulatory incentives for orphan drugs, including tax exemptions and expedited approval processes, which are encouraging pharmaceutical companies to develop treatments for rare diseases.
- Collaborative efforts between local governments and international organizations are enhancing research capabilities and patient access to orphan drugs, particularly in countries like Argentina and Mexico.
North America
- The FDA has accelerated the approval process for orphan drugs, with the Orphan Drug Act providing incentives such as tax credits and market exclusivity, significantly boosting the development of treatments for rare diseases.
- Key players like Vertex Pharmaceuticals and Amgen are investing heavily in research and development for orphan diseases, particularly in gene therapies and biologics, which are expected to revolutionize treatment options.
Middle East And Africa
- The region is witnessing a rise in awareness and advocacy for rare diseases, with organizations like the Middle East Rare Disease Network working to improve diagnosis and treatment options.
- Regulatory bodies in countries such as South Africa are beginning to establish frameworks for orphan drug approval, which is expected to attract investment and improve access to therapies for rare diseases.
Did You Know?
“Approximately 7,000 rare diseases have been identified, yet only about 5% have approved treatments, highlighting a significant unmet medical need in the orphan diseases market.” — National Organization for Rare Disorders (NORD)
Segmental Market Size
The Orphan Diseases Market is a vital segment for addressing the needs of patients with rare conditions for whom there are often inadequate treatment options. This market is currently experiencing growth, primarily driven by greater public awareness of rare diseases and advances in biotechnology. There is also a positive regulatory environment, notably the U.S. Orphan Drug Act, which provides incentives for the development of treatments for these conditions. The current adoption stage of therapies for rare diseases is in the mature phase. The companies leading the way in the development of these treatments include Vertex and Novartis. Gene therapies and biosimilars for rare genetic disorders are the most important applications. Recent examples include treatments for cystic fibrosis and spinal muscular atrophy. The Orphan Diseases Market is also being influenced by the growing trend toward personalization and the increasing focus on rare diseases. The development of CRISPR and next-generation sequencing is enabling the development of more precise and effective treatments for patients with rare diseases.
Future Outlook
Orphan Diseases Market is a promising market with a CAGR of 6.04% from 2023 to 2032. This growth is driven by an increasing prevalence of rare diseases, advances in biotechnology and the growing focus on precision medicine. As regulatory frameworks evolve, particularly with the introduction of incentives for orphan drug development, the number of therapies is expected to increase, thus improving treatment options for patients with rare diseases. Also, the integration of artificial intelligence in drug discovery and the development of gene therapies will contribute to the growth of the market. The collaboration between pharmaceutical companies and academic institutions is expected to lead to the development of novel therapeutics to address unmet medical needs. Awareness and advocacy for rare diseases are also expected to increase, which will lead to increased patient access to treatments, which will in turn increase the penetration of the market. Consequently, by 2032, the market is expected to expand not only in terms of revenue, but also in terms of the diversity of treatments, which will ultimately lead to improved patient outcomes and quality of life.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 11.20% |
Orphan Diseases Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









