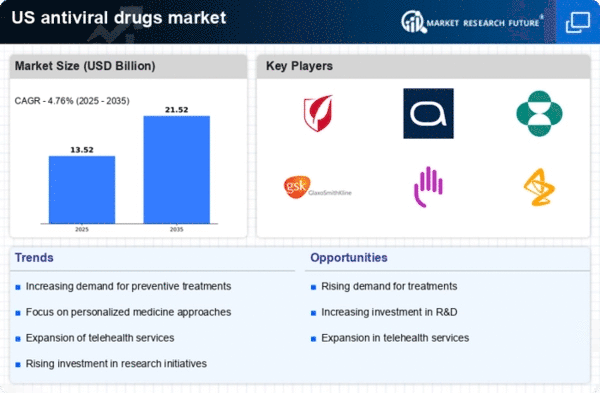
Expansion of Telehealth Services
The expansion of telehealth services in the US is reshaping the landscape of the anti viral-drugs market. With the increasing adoption of telemedicine, patients are more readily accessing healthcare consultations, which facilitates timely diagnosis and treatment of viral infections. This trend is particularly relevant in the context of antiviral therapies, as patients can receive prescriptions for antiviral medications without the need for in-person visits. The convenience and accessibility of telehealth services are likely to enhance patient adherence to antiviral treatment regimens, thereby driving demand within the anti viral-drugs market. As telehealth continues to evolve, it may play a crucial role in improving health outcomes for individuals affected by viral infections.
Rising Incidence of Viral Infections
The increasing prevalence of viral infections in the US is a primary driver for the anti viral-drugs market. Reports indicate that viral infections, such as influenza and hepatitis, have seen a notable rise, leading to heightened demand for effective antiviral therapies. The Centers for Disease Control and Prevention (CDC) has documented that viral infections account for a significant portion of hospitalizations, which underscores the urgent need for innovative antiviral solutions. This trend is likely to propel investments in research and development within the anti viral-drugs market, as pharmaceutical companies strive to address the growing health crisis. Furthermore, the economic burden associated with viral diseases, estimated in billions of dollars annually, further incentivizes stakeholders to prioritize antiviral drug development, thereby fostering market growth.
Growing Awareness of Antiviral Treatments
Public awareness regarding the importance of antiviral treatments is on the rise, which is positively impacting the anti viral-drugs market. Educational campaigns and outreach programs have been instrumental in informing the population about the risks associated with viral infections and the availability of effective antiviral medications. This heightened awareness is reflected in increased patient consultations and prescriptions for antiviral drugs, contributing to market expansion. According to recent surveys, approximately 60% of the population now recognizes the significance of antiviral therapies in managing viral infections. Consequently, healthcare providers are more likely to recommend antiviral treatments, further driving demand within the anti viral-drugs market.
Increased Government Funding for Research
Government initiatives aimed at combating viral diseases are significantly influencing the anti viral-drugs market. In recent years, federal funding for research and development in the field of virology has increased, with allocations reaching upwards of $1 billion annually. This financial support is directed towards fostering innovation in antiviral drug development, enabling researchers to explore novel therapeutic approaches. The National Institutes of Health (NIH) and other governmental bodies are actively promoting collaborations between academia and industry, which is expected to yield breakthroughs in antiviral therapies. As a result, the anti viral-drugs market is likely to experience accelerated growth, driven by the influx of resources dedicated to addressing viral threats and enhancing public health outcomes.
Emergence of Resistance to Existing Antivirals
The emergence of resistance to existing antiviral medications poses a significant challenge, thereby driving the need for new antiviral drugs in the market. As viral pathogens evolve, they may develop resistance to commonly used antiviral agents, necessitating the development of novel therapies. This phenomenon has been observed in various viral infections, including HIV and hepatitis C, where treatment regimens have become less effective over time. The anti viral-drugs market is responding to this challenge by investing in research aimed at discovering and developing next-generation antiviral agents. The potential for lucrative returns on investment in this area is substantial, as healthcare providers seek effective solutions to combat resistant viral strains.
.png)