Growing Awareness and Education
There is a notable increase in awareness and education regarding neurological disorders and their management, which is positively impacting the cerebrospinal fluid-management-devices market. Healthcare professionals are becoming more informed about the latest treatment options and technologies available for managing cerebrospinal fluid-related conditions. This heightened awareness leads to better diagnosis and treatment protocols, ultimately driving demand for effective management devices. Furthermore, patient education initiatives are empowering individuals to seek timely medical intervention, thereby increasing the utilization of cerebrospinal fluid-management devices. As awareness campaigns continue to proliferate, the market is expected to experience growth as more patients and healthcare providers recognize the importance of effective cerebrospinal fluid management.
Increased Healthcare Expenditure
The increasing healthcare expenditure in the US is a significant driver for the cerebrospinal fluid management devices market. With healthcare spending projected to reach approximately $6 trillion by 2027, there is a growing investment in advanced medical technologies. This financial commitment allows for the development and adoption of innovative cerebrospinal fluid management solutions. Hospitals and healthcare facilities are increasingly allocating budgets towards acquiring state-of-the-art devices that enhance patient care and operational efficiency. Additionally, the shift towards value-based care models encourages healthcare providers to invest in technologies that improve patient outcomes while reducing long-term costs. As a result, the cerebrospinal fluid-management-devices market is likely to benefit from this upward trend in healthcare spending.
Supportive Reimbursement Policies
Supportive reimbursement policies play a crucial role in driving the market. Insurance coverage for advanced medical devices is essential for ensuring patient access to necessary treatments. In the US, various insurance providers are increasingly recognizing the importance of cerebrospinal fluid management technologies and are expanding their coverage options. This trend is likely to encourage healthcare providers to adopt these devices, knowing that patients will have financial support for their treatments. Additionally, favorable reimbursement rates can stimulate innovation within the market, as manufacturers are incentivized to develop new and improved devices. As reimbursement policies continue to evolve, the cerebrospinal fluid-management-devices market is expected to thrive.
Rising Incidence of Neurological Disorders
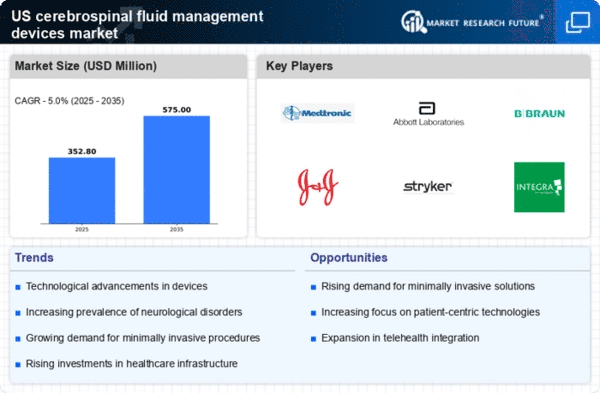
The increasing prevalence of neurological disorders in the US is a primary driver for the cerebrospinal fluid-management-devices market. Conditions such as hydrocephalus, multiple sclerosis, and traumatic brain injuries are becoming more common, necessitating effective management solutions. According to recent data, the incidence of hydrocephalus alone is estimated to affect approximately 1 in 1,000 live births, leading to a growing demand for cerebrospinal fluid management devices. This trend indicates a potential market growth as healthcare providers seek advanced technologies to address these conditions. Furthermore, the aging population is likely to exacerbate this issue, as older adults are more susceptible to neurological disorders. Consequently, the cerebrospinal fluid-management-devices market is poised for expansion as healthcare systems adapt to these rising challenges.
Technological Innovations in Device Design
Innovations in the design and functionality of cerebrospinal fluid-management devices are significantly influencing the market. Recent advancements include the development of smart shunts and programmable valves that enhance patient outcomes and reduce complications. These innovations not only improve the efficiency of fluid management but also minimize the need for invasive procedures. The introduction of minimally invasive techniques is expected to drive market growth, as they offer patients quicker recovery times and lower risks of infection. Moreover, the integration of telemedicine and remote monitoring capabilities into these devices is likely to enhance patient management, further propelling the cerebrospinal fluid-management-devices market. As technology continues to evolve, the demand for sophisticated devices that provide real-time data and improved patient care is anticipated to rise.