US Drug Repurposing Market Overview
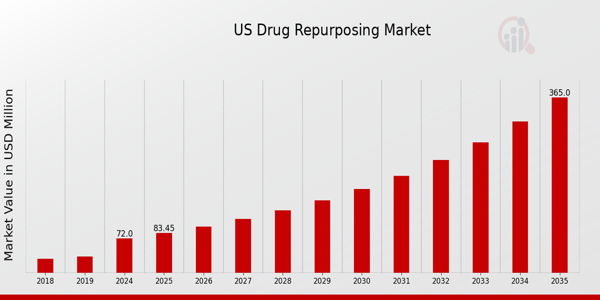
As per MRFR analysis, the US Drug Repurposing Market Size was estimated at 63 (USD Million) in 2023.The US Drug Repurposing Market Industry is expected to grow from 72(USD Million) in 2024 to 365 (USD Million) by 2035. The US Drug Repurposing Market CAGR (growth rate) is expected to be around 15.901% during the forecast period (2025 - 2035)
Key US Drug Repurposing Market Trends Highlighted
The US Drug Repurposing Market is experiencing several notable trends, driven largely by advancements in technology and the evolving landscape of healthcare. One key market driver is the increasing focus on cost-effectiveness and the need for faster drug development processes. With the rising costs of bringing new drugs to market, pharmaceutical companies are leveraging existing drugs for new applications, thereby significantly shortening development timelines. Regulatory bodies like the FDA are also playing a crucial role by encouraging drug repurposing through streamlined approval processes, which enable quicker access to therapies for patients.
Opportunities for capturing market share are growing as public and private sectors invest in research initiatives focused on repurposing. Collaborations between academia and the pharmaceutical sector are becoming more common, fostering innovation and accelerating discovery. The recent surge in interest in drugs considered for repurposing due to the COVID-19 pandemic has further illuminated the potential for existing medications to provide new treatments for various conditions. Additionally, there is an increasing emphasis on data-driven approaches in the US Drug Repurposing Market, with artificial intelligence and machine learning being utilized to identify potential candidates for repurposing.
This trend showcases how technology can enhance drug discovery and optimize the treatment pipeline. Furthermore, patient advocacy groups are becoming more vocal in promoting the benefits of repurposed drugs, increasing awareness and acceptance among healthcare providers. Overall, the convergence of cost pressures, regulatory support, technological advancements, and patient advocacy is shaping a dynamic environment in the US Drug Repurposing Market.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
US Drug Repurposing Market Drivers
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic illnesses, including diabetes, cancer, and cardiovascular disorders in the general population, is a major factor propelling the US drug repurposing market industry. About 6 out of 10 people in the US have a chronic illness, and 4 out of 10 have two or more, according to the Centers for Disease Control and Prevention (CDC). Due to the pressing demand for efficient therapies brought on by the rise in chronic illnesses, scientists and pharmaceutical corporations are looking into medication repurposing as a potential way to swiftly develop new therapeutic choices.
Large pharmaceutical companies like Pfizer and Johnson & Johnson are spending more money on R&D projects that aim to repurpose current medications for novel therapeutic uses. This strategy highlights the industry's development potential in the upcoming years by cutting down on the time to market as well as the total expenses related to introducing new medications into clinical use.
Advancements in Computational Drug Discovery
Modern advancements in computational drug discovery and artificial intelligence (AI) are fueling growth in the US Drug Repurposing Market Industry. Increased computational power allows for the analysis of vast datasets, leading to the identification of new uses for existing drugs. The National Institutes of Health (NIH) has reported a 200% increase in the use of computational techniques in drug repurposing studies over the last five years. Companies such as IBM, with its Watson for Drug Discovery, are leveraging AI to uncover potential new uses for existing medications.
These advancements enable faster identification of candidates for repurposing, thus accelerating the process of bringing treatments to patients, showcasing a significant driver for market expansion.
Growing Investment in Drug Repurposing Initiatives
The rise in funding for drug repurposing initiatives plays a vital role in the US Drug Repurposing Market Industry. Government entities and venture capital firms are increasingly providing financial support for research aimed at repurposing existing drugs. For instance, the National Institutes of Health announced a substantial federal grant program aimed at repurposing drugs for community-level health challenges, which has already allocated over USD 200 million since its inception.
This financial backing encourages academic institutions and pharmaceutical companies to collaborate on repurposing projects, leading to innovative treatments that address urgent health needs efficiently. As a result, this increasing level of investment is propelling the market forward.
Regulatory Support for Drug Repurposing
A favorable regulatory environment supporting drug repurposing significantly contributes to the growth of the US Drug Repurposing Market Industry. Regulatory bodies like the Food and Drug Administration (FDA) have established programs such as the Orphan Drug Designation and the Fast Track designation, which expedite the review process for repurposed drugs. In recent years, the FDA has received a notable increase in repurposed drug applications, with over 30% of new drug approvals in the last decade involving previously approved therapies.
This level of regulatory encouragement not only streamlines the approval process but also enhances the attractiveness of drug repurposing strategies, further establishing it as a key driver for the market.
US Drug Repurposing Market Segment Insights
Drug Repurposing Market Types Insights
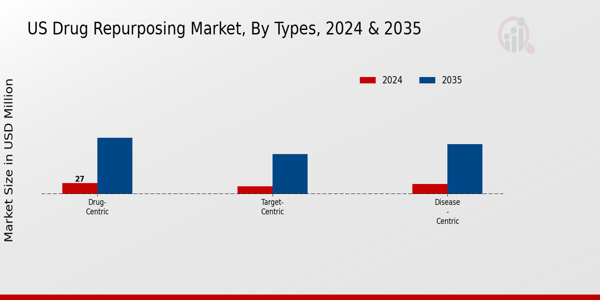
The US Drug Repurposing Market has gained significant attention due to its potential to expedite treatment options and optimize the existing drug pipeline. This market can be broadly classified into three primary types: Disease-centric, Target-centric, and Drug-centric approaches. The Disease-centric type focuses on identifying existing medications that can be utilized to treat different conditions than originally intended, often leading to breakthroughs in therapies for chronic and complex diseases. This approach is pivotal as it leverages existing data and safety profiles, potentially shortening the time for new treatments to reach patients.
The Target-centric approach targets specific biological mechanisms underlying diseases, allowing pharmaceuticals to explore alternative therapies quickly and efficiently by repurposing existing drugs that interact with certain pathways. This type of approach is significant for driving innovation in precision medicine, especially in areas with high unmet medical needs. Meanwhile, the Drug-centric approach centers on repurposing drugs to treat new indications, highlighting the adaptability of compounds that have already undergone extensive testing for safety and efficacy.
Given that the US is a leading hub for pharmaceutical R&D, these approaches support the growing market, spurred by favorable governmental policies and funding initiatives aimed at enhancing drug repurposing strategies. The overall surge in adoption of these strategies is driven by the public's demand for more rapid solutions to emerging health crises, such as antiviral therapies in response to pandemics, thus reinforcing the significance of the Drug Repurposing Market in the US healthcare landscape. Each type within the segmentation presents unique opportunities, challenges, and methodologies that cater to a variety of therapeutic needs, ultimately contributing to a more responsive healthcare system.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Drug Repurposing Market Therapeutic Area Insights
The US Drug Repurposing Market within the Therapeutic Area segment is rapidly evolving, driven by the increasing demand for innovative and cost-effective treatment solutions. This segment encompasses various approaches, notably Same Therapeutic Area and Different Therapeutic Area strategies. The Same Therapeutic Area approach focuses on identifying new uses for existing drugs that target the same diseases, contributing to enhanced treatment protocols and patient outcomes while minimizing Research and Development costs. Conversely, the Different Therapeutic Area strategy expands the scope of drug applications to treat entirely distinct diseases, leveraging previously established safety profiles to streamline the development process.
This diversification is gaining traction as healthcare systems strive for improved efficiency and better resource allocation. Coupled with increasing regulatory support for repurposed drugs, these strategies position themselves as vital components in addressing unmet medical needs in the US. With a clear focus on therapeutic efficacy and safety, Drug Repurposing emerges as a promising avenue to optimize healthcare delivery and improve patient access to effective treatments. The ongoing evolution of this market is expected to provide significant opportunities for growth and innovation in the coming years.
Drug Repurposing Market Drug Molecules Insights
The 'US Drug Repurposing Market', focusing on Drug Molecules encompasses various crucial components that drive innovation in therapeutic applications. Biologics and Small Molecules are the primary contributors within this segment, catering to different medical needs. Biologics, derived from living organisms, are essential for treating complex diseases, including autoimmune disorders and certain types of cancers, thus showing substantial growth due to their targeted approach. Small Molecules dominate the pharmaceutical landscape, being crucial for a broad spectrum of diseases, including infectious diseases and chronic conditions, given their ability to penetrate cells easily.
The growth in Drug Molecules is supported by advancements in Research and Development, enhanced regulatory approval processes, and a growing shift towards personalized medicine. Moreover, the rising prevalence of various diseases and the need for cost-effective treatment options further drive market dynamics, highlighting the significance of Drug Molecules. The ongoing trend in repurposing existing drugs is expected to accelerate the pace of delivery and reduce time to market, making the Drug Molecules segment a vital area in the US Drug Repurposing Market.
US Drug Repurposing Market Key Players and Competitive Insights
The US Drug Repurposing Market is experiencing significant growth, characterized by an increasing number of pharmaceutical companies and research institutions exploring new therapeutic applications for existing medications. This market is driven by the potential for quicker, more cost-effective drug development compared to traditional drug discovery methods, which often involve lengthy and expensive processes.
Competitive insights reveal that numerous organizations are actively pursuing repurposing strategies, which capitalize on already established safety profiles of drugs, thereby allowing them to identify new treatment options for various diseases, including rare and complex conditions. As innovation in biotechnology and pharmaceuticals continues to grow, the landscape of drug repurposing signifies a paradigm shift, encouraging collaborations among various stakeholders, including academia, government agencies, and the private sector.
AstraZeneca is a prominent player in the US Drug Repurposing Market, leveraging its extensive research capabilities and robust pipeline of pharmaceuticals. The company is well-known for its commitment to innovation, which plays a crucial role in its drug repurposing efforts. AstraZeneca has a strong foundation built on significant therapeutic expertise across multiple disease areas, such as oncology, cardiovascular, and respiratory diseases, enabling it to identify new indications for existing drugs.
With a focus on strategic partnerships and collaborations, AstraZeneca maximizes its resources and expands its reach in discovering novel usages for established products, an approach that enhances its competitive edge in the market. The company’s reputation for prioritizing patient needs underlies its strategies in determining how to best reposition drugs effectively within the US market.
Merck is another key player in the US Drug Repurposing Market, known for its established portfolio of pharmaceuticals and strong emphasis on research and development. The company has made substantial investments in finding new therapeutic applications for its existing drugs, aiming to promote advancements in treatment options. Merck’s key products already include several that have shown potential for repurposing, further solidifying its position within the market. By focusing on therapeutic areas such as infectious diseases and oncology, Merck has been able to leverage its scientific expertise to revitalize older compounds.
The company actively pursues strategic mergers and acquisitions to bolster its capabilities in drug repurposing, thereby enhancing its market presence and fortifying its strengths in delivering innovative healthcare solutions. Through its dedication to continuous exploration and application of existing medicines, Merck contributes significantly to the dynamic landscape of the US Drug Repurposing Market.
Key Companies in the US Drug Repurposing Market Include
- AstraZeneca
- Merck
- Roche
- Amgen
- Bristol Myers Squibb
- Sanofi
- Eli Lilly
- Johnson & Johnson
- Regeneron Pharmaceuticals
- Gilead Sciences
- AbbVie
- Novartis
- Pfizer
- Vertex Pharmaceuticals
US Drug Repurposing Market Industry Developments
Significant developments in the US Drug Repurposing Market have emerged recently, particularly driven by large pharmaceutical companies such as AstraZeneca, Merck, and Roche, focusing on the potential of existing drugs for new therapeutic uses. In October 2023, Amgen announced a substantial partnership with a biotech firm to advance its drug repurposing initiatives, targeting conditions like neurodegenerative diseases. Furthermore, Bristol Myers Squibb has been actively exploring the potential of its existing oncology drugs to treat autoimmune disorders, demonstrating a growing trend in the industry. Notably, Eli Lilly reported a successful trial of a repurposed diabetes medication for treating Alzheimer's disease in August 2023, signaling a pivotal shift in therapeutic applications.
The market valuation for US Drug Repurposing continues to rise, fueled by innovations and cost-cutting strategies associated with leveraging existing therapies. Additionally, in September 2023, Regeneron Pharmaceuticals acquired a small company specializing in repurposing therapies for genetic disorders, enhancing its pipeline. Overall, the US Drug Repurposing Market remains vibrant, with ongoing investments and strategic collaborations among major players like Johnson and Johnson, Gilead Sciences, and AbbVie, fostering a robust environment for drug innovation.
Drug Repurposing Market Segmentation Insights
- Drug Repurposing Market Types Outlook
- Disease-centric
- Target-centric
- Drug-centric
- Drug Repurposing Market Therapeutic Area Outlook
- Same Therapeutic Area
- Different Therapeutic Area
- Drug Repurposing Market Drug Molecules Outlook
| Report Attribute/Metric |
Details |
| Market Size 2023 |
63.0(USD Million) |
| Market Size 2024 |
72.0(USD Million) |
| Market Size 2035 |
365.0(USD Million) |
| Compound Annual Growth Rate (CAGR) |
15.901% (2025 - 2035) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Base Year |
2024 |
| Market Forecast Period |
2025 - 2035 |
| Historical Data |
2019 - 2024 |
| Market Forecast Units |
USD Million |
| Key Companies Profiled |
AstraZeneca, Merck, Roche, Amgen, Bristol Myers Squibb, Sanofi, Eli Lilly, Johnson & Johnson, Regeneron Pharmaceuticals, Gilead Sciences, AbbVie, Novartis, Pfizer, Vertex Pharmaceuticals |
| Segments Covered |
Types, Therapeutic Area, Drug Molecules |
| Key Market Opportunities |
Accelerated FDA approval processes, Increased focus on rare diseases, Cost-effective therapeutic solutions, Expanding applications of AI, and Growing interest from pharmaceutical companies |
| Key Market Dynamics |
increasing healthcare costs, accelerating drug development timelines, growing prevalence of chronic diseases, rising interest in personalized medicine, expanding regulatory support for repurposing |
| Countries Covered |
US |
Frequently Asked Questions (FAQ) :
The US Drug Repurposing Market is expected to be valued at 72.0 million USD in 2024.
By 2035, the US Drug Repurposing Market is anticipated to reach a valuation of 365.0 million USD.
The market is projected to grow at a CAGR of 15.901% from 2025 to 2035.
The major types in the market include Disease-centric, Target-centric, and Drug-centric segments.
The Disease-centric segment is valued at 25.0 million USD in 2024.
The Drug-centric segment is expected to be valued at 140.0 million USD by 2035.
The Target-centric segment is expected to be valued at 20.0 million USD in 2024.
Major players include AstraZeneca, Merck, Roche, Amgen, and Bristol Myers Squibb among others.
The Drug-centric segment is projected to grow significantly, reaching 140.0 million USD by 2035.
Key trends include increasing investment in research and development and a focus on faster drug approval processes.
















