US Epilepsy Devices Market Overview
As per MRFR analysis, the US Epilepsy Devices Market Size was estimated at 183.75 (USD Million) in 2023.The US Epilepsy Devices Market Industry is expected to grow from 193.55 (USD Million) in 2024 to 495 (USD Million) by 2035. The US Epilepsy Devices Market CAGR (growth rate) is expected to be around 8.912% during the forecast period (2025 - 2035)
Key US Epilepsy Devices Market Trends Highlighted
The US Epilepsy Devices Market is experiencing a number of important trends that reflect advancements in technology and an increase in awareness surrounding epilepsy. Key market drivers include the rising prevalence of epilepsy and the growing need for effective management solutions. The Centers for Disease Control and Prevention (CDC) indicate that nearly 1.2% of the population in the US suffers from active epilepsy, propelling a demand for innovative devices. There is a particular focus on devices like responsive neurostimulation systems and wearable monitors, which cater to the needs of patients seeking more reliable management options.
Additionally, opportunities to be explored in this market include the integration of digital health technologies and telemedicine into epilepsy care. There is a tremendous opportunity for device manufacturers to create new products and services that better assist patients. Recently, there has been a shift towards more patient-centered methodologies, which is increasing the development of more personalized devices for specific patients. Activities from advocates have also raised the awareness and acceptance of epilepsy so more people are willing to seek help.
Therefore, innovation will be aimed at improving the ease of use and living with devices that people with epilepsy in the US have to use.The combination of these trends and developments is shaping a dynamic environment in the US Epilepsy Devices Market, signaling a promising future for patients and healthcare providers alike.
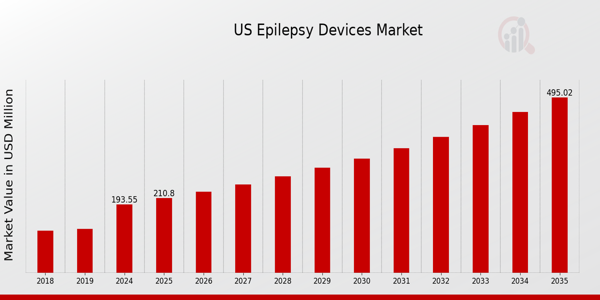
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
US Epilepsy Devices Market Drivers
Increasing Prevalence of Epilepsy in the United States
The rising number of epilepsy cases in the United States is a significant driver for the US Epilepsy Devices Market Industry. According to the Centers for Disease Control and Prevention (CDC), approximately 3.4 million individuals in the United States are affected by epilepsy, with the incidence of new cases estimated at 150,000 annually. This growing patient population is leading to an increased demand for innovative epilepsy devices aimed at improving the quality of life for patients and enhancing seizure management.
Furthermore, the National Institutes of Health (NIH) emphasizes the need for effective treatment solutions, encouraging further Research and Development (R&D) in the field. The strategic focus on enhancing the existing treatment landscape by organizations such as the Epilepsy Foundation is aligned with the rising prevalence, making patient-centric devices increasingly vital to meet this urgent healthcare requirement.
Technological Advancements in Epilepsy Devices
The advent of advanced technologies in the US Epilepsy Devices Market Industry is rapidly transforming the landscape of epilepsy management. Organizations such as Medtronic and Abbott Laboratories are introducing cutting-edge devices, including responsive neurostimulation techniques and implantable devices that significantly improve seizure detection and response times. The development of more sophisticated algorithms to analyze electroencephalogram (EEG) data allows for personalized treatment plans, further boosting market growth.
The FDA's recent approval of various innovative epilepsy devices is indicative of a supportive regulatory environment catalyzing advancement in technology, ensuring better patient outcomes, and attracting investments in this sector.
Growing Awareness and Education on Epilepsy
There is a noticeable increase in awareness and education surrounding epilepsy, which directly influences the US Epilepsy Devices Market Industry. The Epilepsy Foundation and similar organizations are actively campaigning to educate the public about epilepsy, aiming to reduce stigma and improve treatment accessibility. As a result of these efforts, more individuals are seeking diagnosis and treatment options, significantly increasing the market for epilepsy devices.
Additionally, government initiatives have emphasized public health campaigns that promote understanding of seizure disorders, leading to a higher demand for devices that assist in epilepsy management and tracking.
Healthcare Policy Changes Favoring Epilepsy Treatment
Recent healthcare policy changes in the United States have created a favorable environment for the US Epilepsy Devices Market Industry. Policies that expand healthcare coverage for patients with chronic conditions, including epilepsy, are paving the way for increased access to advanced medical devices. The Affordable Care Act (ACA) has expanded insurance coverage, ensuring more epilepsy patients can access innovative treatment options.
This legislative push has resulted in a more significant number of therapies being covered, effectively increasing the demand for epilepsy devices within the market.The collaboration between government healthcare programs and private organizations further enhances accessibility, driving growth in the epilepsy device segment.
US Epilepsy Devices Market Segment Insights
Epilepsy Devices Market Product Type Insights
The US Epilepsy Devices Market is characterized by a diverse array of product types, each playing a crucial role in managing epilepsy. Among these, Conventional Devices, which include traditional monitoring and therapeutic tools, have long been the mainstream approach for treatment, providing foundational support for patients. Wearable Devices have gained significant traction, driven by advancements in technology and the growing emphasis on remote patient monitoring. These devices facilitate continuous tracking of seizure activity and can improve patient outcomes by offering real-time data to healthcare providers.
Implantable Devices, such as neurostimulators, represent a promising area within the market, as they directly interact with the nervous system to reduce seizure frequency, thereby providing patients with new avenues for treatment. The Diet segment, which comprises therapeutic diets like the ketogenic diet, plays a complementary role in epilepsy management by empowering patients through lifestyle modifications alongside device usage. Additionally, other related technologies and methods, capturing various innovative approaches to treatment, fill out the landscape of the Epilepsy Devices Market.
The interplay among these product types illustrates a growing trend toward personalized medicine and integrated therapy approaches that incorporate both devices and lifestyle changes for effective seizure management. Overall, the market segmentation highlights the importance of a multi-faceted strategy to address the diverse needs of individuals living with epilepsy in the US. Through a combination of conventional, innovative, dietary, and various other solutions, the Epilepsy Devices Market continues to evolve.
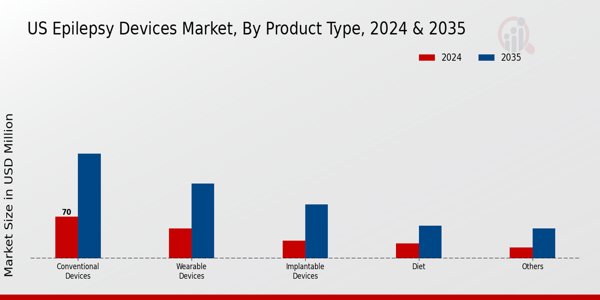
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Epilepsy Devices Market Technology Insights
The Technology segment of the US Epilepsy Devices Market showcases a diverse range of innovative therapeutic and monitoring options tailored to enhance the quality of life for individuals with epilepsy. Vagus Nerve Stimulation has emerged as a prominent method for managing seizures, providing continuous stimulation to the vagus nerve, which can lead to a significant reduction in seizure frequency for many patients. Deep Brain Stimulation is gaining traction as well, particularly among patients who do not respond adequately to traditional treatments, as this technology can target specific brain regions involved in seizure activity to modulate and improve outcomes.
Accelerometry, which involves the use of wearable devices, offers real-time monitoring of seizure activity through movement detection, allowing for better patient management and data collection. Responsive Neurostimulation serves as a cutting-edge solution that enables the device to detect abnormal electrical activity in the brain and deliver targeted pulses, thus preventing seizures before they occur. Other emerging technologies continue to develop, focusing on alternative approaches to seizure management, which could enhance treatment personalization. As the US Epilepsy Devices Market progresses, increased technological advancements and integration of data analytics will likely empower patients and caregivers with improved tools for managing epilepsy effectively.
Epilepsy Devices Market End Users Insights
The End Users segment of the US Epilepsy Devices Market encompasses various healthcare settings playing crucial roles in patient management and care. Hospitals and Clinics serve as primary centers for diagnosis and treatment, offering advanced medical technologies and expertise in seizure management, while Neurology Centers specialize in neurological disorders, providing tailored care and support for epilepsy patients. Home Care Settings reflect a growing trend towards personalized care, accommodating patients who prefer to manage their conditions from the comfort of home, thereby enhancing their quality of life.
Additionally, the category of Others captures diverse applications, including rehabilitation centers and telemedicine platforms, which are increasingly significant for ongoing patient monitoring and support. The rising prevalence of epilepsy and increasing awareness around seizure management drive demand across these settings, contributing to the overall market growth. Enhanced technological advancements in device development further fuel market opportunities as healthcare providers strive to improve patient outcomes through innovative solutions. This segmentation illustrates the importance of various care environments and their collaborative roles in the holistic management of epilepsy within the US, effectively addressing the diverse needs of patients and healthcare professionals alike.
US Epilepsy Devices Market Key Players and Competitive Insights
The US Epilepsy Devices Market is a dynamic and rapidly evolving sector that plays a critical role in addressing the needs of individuals suffering from epilepsy. The competitive landscape is characterized by a variety of key players who are actively engaged in the development of innovative technologies aimed at improving patient outcomes. These companies focus on a range of devices, including neurostimulation devices, medication delivery systems, and monitoring tools. The market's growth is driven by increasing awareness of epilepsy and advancements in technology that facilitate better diagnosis and management of the disorder.
Competitive strategies often involve collaborations, mergers, and acquisitions, as well as significant investment in research and development to create effective solutions and enhance market presence. Abbott stands out in the US Epilepsy Devices Market due to its strong portfolio of advanced neurostimulation technologies that are designed to treat epilepsy by minimizing seizures and improving the quality of life for patients. The company has established a significant market presence through its innovative products and strong brand reputation. Abbott's strengths lie in its commitment to research and development, which enables the continual enhancement of its devices and offerings.
Utilizing a patient-centric approach, Abbott focuses on understanding patient needs and tailoring solutions accordingly, which has contributed to its competitive edge. The company also benefits from strategic partnerships and collaborations, enhancing its reach and influence within the epilepsy treatment segment. LivaNova plays a vital role in the US Epilepsy Devices Market with its unique offerings, particularly in the area of neuromodulation for epilepsy treatment. Key products include advanced responsive neurostimulation devices that target seizure control and improve the overall management of epilepsy.
LivaNova's market presence is bolstered by its ongoing commitment to innovation and customer service, which positions it favorably against competitors. The company emphasizes research initiatives, which often lead to breakthrough therapies that enhance its product line. Furthermore, LivaNova has engaged in mergers and acquisitions to expand its capabilities and product offerings in the US, thereby increasing its market share and strengthening its competitive standing. The combination of innovative technology and strategic growth initiatives underpins LivaNova's robust presence in the arena of epilepsy devices in the United States.
Key Companies in the US Epilepsy Devices Market Include
US Epilepsy Devices Market Industry Developments
The US Epilepsy Devices Market has seen several significant developments recently. In December 2022, Medtronic received FDA approval for its Percept PC device, which utilizes neurostimulation technology to detect seizures. In January 2023, Abbott launched its new neuropace platform designed to provide advanced seizure management tools. Furthermore, Boston Scientific announced in February 2023 a partnership with NeuroPace to enhance its epilepsy monitoring solutions. The market has also experienced notable growth, with reports indicating an increase of over 10% in market valuation year-on-year, driven by rising demand for innovative therapies and devices.
In terms of mergers and acquisitions, LivaNova completed its acquisition of the neuromodulation business from Intent Solutions in March 2023, expanding its portfolio significantly. This acquisition aligns with the trend of consolidation in the industry to enhance product offerings in medical devices for epilepsy. Key players, including Natus Medical and Stryker, continue to innovate, with investment in Research and Development aimed at improving patient outcomes. Over the past few years, advancements in technology and regulatory approvals have markedly influenced the landscape of the epilepsy devices market in the US, indicating a strong future trajectory for the industry.
Epilepsy Devices Market Segmentation Insights
-
Epilepsy Devices Market Product Type Outlook
- Wearable Devices
- Implantable Devices
- Diet
- Others
-
Epilepsy Devices Market Technology Outlook
- Deep Brain Stimulation
- Accelerometery
- Responsive Neurostimulation
- Others
| Report Attribute/Metric |
Details |
| Market Size 2023 |
183.75 (USD Million) |
| Market Size 2024 |
193.55 (USD Million) |
| Market Size 2035 |
495.0 (USD Million) |
| Compound Annual Growth Rate (CAGR) |
8.912% (2025 - 2035) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Base Year |
2024 |
| Market Forecast Period |
2025 - 2035 |
| Historical Data |
2019 - 2024 |
| Market Forecast Units |
USD Million |
| Key Companies Profiled |
Abbott, LivaNova, Civitas Solutions, Medtronic, Boston Scientific, Natus Medical, Compumedics, Cerecor, Stryker, Insmed, Zebra Medical Vision, Reflect Scientific, NeuroPace, ElectroCore |
| Segments Covered |
Product Type, Technology, End Users |
| Key Market Opportunities |
Remote monitoring technologies, Wearable seizure detection devices, Neuromodulation therapy innovations, Enhanced patient education solutions, Telemedicine integration for care |
| Key Market Dynamics |
Increasing prevalence of epilepsy, Technological advancements in devices, Growing awareness and diagnosis, Rising healthcare expenditure, Government support and funding |
| Countries Covered |
US |
Frequently Asked Questions (FAQ):
The US Epilepsy Devices Market is expected to be valued at 193.55 million USD in 2024.
By 2035, the US Epilepsy Devices Market is projected to reach a value of 495.0 million USD.
The market is expected to grow at a CAGR of 8.912% from 2025 to 2035.
Conventional devices are expected to lead the market with a valuation of 70.0 million USD in 2024.
Wearable devices are projected to reach a market value of 125.0 million USD by 2035.
Major players in the market include Abbott, LivaNova, Medtronic, and Boston Scientific, among others.
Implantable devices are estimated to be valued at 30.0 million USD in 2024.
The market may encounter challenges such as regulatory hurdles and competition from alternative therapies.
Growth rates are anticipated to vary, with implantable devices growing significantly due to technological advancements.
Opportunities for growth include advancements in technology and increasing awareness and diagnosis of epilepsy.
















