Growing Patient Support Networks
The establishment of growing patient support networks plays a pivotal role in the evans syndrome market. These networks provide essential resources, education, and advocacy for individuals affected by Evans syndrome. By fostering a sense of community, they empower patients to seek timely diagnosis and treatment, which can lead to improved health outcomes. Additionally, these organizations often collaborate with healthcare professionals and researchers to raise awareness about the condition, thereby driving demand for effective therapies. As more patients engage with these support networks, the visibility of Evans syndrome increases, potentially leading to greater funding for research and development. This dynamic interaction between patients and support networks is likely to catalyze growth in the evans syndrome market.
Increased Healthcare Expenditure
The rising healthcare expenditure in the US is significantly impacting the evans syndrome market. With healthcare spending projected to reach approximately $4 trillion by 2025, there is a growing focus on rare diseases and their management. This increase in funding allows for better diagnostic tools, treatment options, and patient support services. Furthermore, as healthcare policies evolve to prioritize rare diseases, more resources are allocated to research and development in the evans syndrome market. This financial commitment from both public and private sectors is likely to enhance the availability of therapies and improve patient access to care. Consequently, the evans syndrome market stands to benefit from this upward trend in healthcare investment.
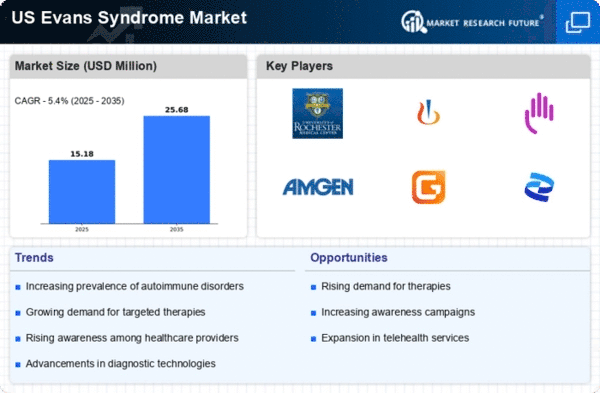
Rising Incidence of Evans Syndrome
The increasing incidence of Evans syndrome in the US is a notable driver for the evans syndrome market. Recent estimates suggest that the prevalence of this rare autoimmune disorder is on the rise, with approximately 1 in 100,000 individuals affected. This growing patient population necessitates enhanced healthcare services and treatment options, thereby expanding the market. As awareness of the condition improves, more patients are likely to seek diagnosis and treatment, further contributing to market growth. The rising incidence also prompts healthcare providers to invest in research and development, leading to innovative therapies. Consequently, The Evans syndrome market is expected to experience significant growth as healthcare systems adapt to meet the needs of this increasing patient demographic.
Innovative Therapeutic Developments
The emergence of innovative therapeutic developments is a critical driver for the evans syndrome market. Recent advancements in biologics and targeted therapies have shown promise in treating autoimmune disorders, including Evans syndrome. For instance, therapies that modulate the immune response are gaining traction, potentially improving patient outcomes. The market is witnessing a surge in clinical trials aimed at evaluating the efficacy of these novel treatments, with several candidates currently in various stages of development. This influx of innovative therapies not only enhances treatment options but also attracts investment from pharmaceutical companies, thereby stimulating market growth. As these therapies receive regulatory approval, the evans syndrome market is poised for expansion, offering new hope to patients and healthcare providers alike.
Regulatory Support for Rare Diseases
Regulatory support for rare diseases is emerging as a significant driver for the evans syndrome market. In recent years, the US government has implemented various initiatives aimed at expediting the approval process for treatments targeting rare conditions. Programs such as the Orphan Drug Act provide incentives for pharmaceutical companies to develop therapies for diseases like Evans syndrome. This regulatory environment encourages innovation and investment in the market, as companies are more likely to pursue research and development for rare diseases with the promise of market exclusivity and financial incentives. As a result, the evans syndrome market is likely to see an influx of new therapies and improved patient access to care, driven by this supportive regulatory framework.
















Leave a Comment