Rising Diabetes Prevalence
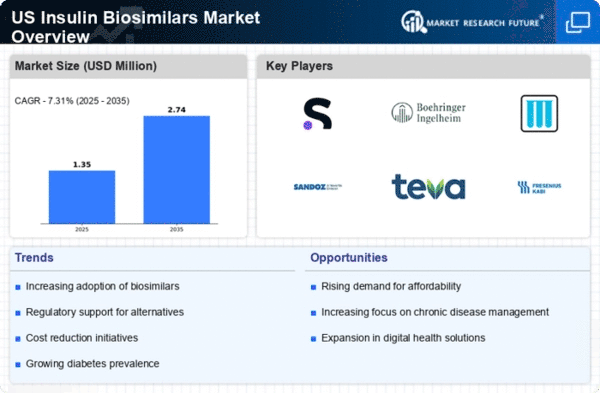
The increasing incidence of diabetes in the US is a primary driver for the insulin biosimilars market. According to the Centers for Disease Control and Prevention (CDC), approximately 34.2 million people in the US have diabetes, which represents about 10.5% of the population. This growing patient base necessitates a more extensive range of insulin products, including biosimilars, to meet the demand for affordable treatment options. As healthcare costs continue to rise, the insulin biosimilars market is likely to expand, providing patients with more accessible alternatives. Furthermore, the increasing awareness of diabetes management and the importance of insulin therapy is expected to further propel the market, as patients seek effective and cost-efficient solutions for their treatment.
Cost Containment Initiatives
Cost containment measures implemented by healthcare providers and insurers are significantly influencing the insulin biosimilars market. With the rising costs of diabetes management, stakeholders are increasingly looking for ways to reduce expenses. Biosimilars, which are typically priced lower than their reference biologics, offer a viable solution. The US government has also introduced various policies aimed at reducing drug prices, which may further encourage the adoption of biosimilars. For instance, the introduction of the Affordable Care Act has led to increased scrutiny of drug pricing, prompting insurers to favor lower-cost alternatives. As a result, the insulin biosimilars market is likely to benefit from these initiatives, as patients and providers alike seek more economical treatment options.
Regulatory Framework Enhancements
The evolving regulatory framework surrounding biosimilars is shaping the insulin biosimilars market. The US Food and Drug Administration (FDA) has established a clear pathway for the approval of biosimilars, which has encouraged manufacturers to invest in this sector. Recent guidelines have streamlined the approval process, making it easier for new entrants to bring their products to market. This regulatory support is crucial, as it fosters innovation and competition within the insulin biosimilars market. Additionally, the FDA's commitment to ensuring the safety and efficacy of biosimilars reassures both healthcare providers and patients, potentially increasing acceptance and utilization of these products. As the regulatory landscape continues to evolve, it is expected that the insulin biosimilars market will experience further growth.
Patient Demand for Affordable Options
The demand for affordable insulin options is a critical driver of the insulin biosimilars market. Many patients face financial barriers when accessing insulin, leading to non-adherence to prescribed therapies. A survey conducted by the American Diabetes Association revealed that nearly 1 in 4 insulin users reported rationing their insulin due to cost concerns. This situation has prompted a push for more affordable alternatives, such as biosimilars, which can provide similar efficacy at a lower price point. As patients advocate for their rights to access necessary medications, the insulin biosimilars market is likely to see increased uptake, as healthcare providers respond to this demand by incorporating biosimilars into treatment regimens.
Increased Investment in Biosimilar Development
Investment in the development of biosimilars is on the rise, driven by the potential for high returns in the insulin biosimilars market. Pharmaceutical companies are recognizing the lucrative opportunities presented by biosimilars, particularly as patents for original insulin products expire. The US market is witnessing a surge in research and development activities, with numerous companies entering the biosimilars space. According to the FDA, there are currently over 50 biosimilars approved for various indications, with insulin biosimilars being a key focus area. This influx of investment is expected to enhance competition, leading to improved product offerings and potentially lower prices for consumers. As more players enter the market, the insulin biosimilars market is poised for substantial growth.