US IV Fluid Monitoring Devices Market Research Report By End-user (Hospitals and clinics, ASCs, Home care) and By Type (Desktop, Portable) - Forecast to 2035
- ID: MRFR/MED/18416-HCR
- | Pages: 100
- | Author: Garvit Vyas
- | Publish Date: Sep 2025


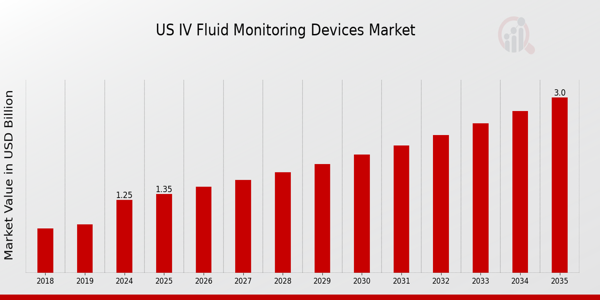
As per MRFR analysis, the US IV Fluid Monitoring Devices Market Size was estimated at 1.1 (USD Billion) in 2023. The US IV Fluid Monitoring Devices Market Industry is expected to grow from 1.25(USD Billion) in 2024 to 3 (USD Billion) by 2035. The US IV Fluid Monitoring Devices Market CAGR (growth rate) is expected to be around 8.284% during the forecast period (2025 - 2035).
The US IV Fluid Monitoring Devices Market is witnessing significant trends driven by advancements in technology and an increasing focus on patient safety. The surge in chronic diseases requiring fluid therapy, such as heart failure and diabetes, has heightened the importance of accurate monitoring. Additionally, there is a growing emphasis on reducing human error in IV administration, leading to the adoption of automated IV fluid monitoring systems.
The incorporation of smart technologies and real-time data analytics in these devices is becoming essential to ensure optimal dosing and enhance patient outcomes. Opportunities in the US market include the potential for developing portable and easy-to-use monitoring devices that can be utilized in various healthcare settings, from hospitals to home healthcare. As the aging population in the US grows, there is an increasing demand for innovative and user-friendly IV fluid monitoring solutions that cater to the home healthcare sector, providing comfort and independence to patients.
Moreover, government initiatives aimed at improving healthcare infrastructure and investment in medical technology are further propelling the growth of this market segment. Recent trends indicate a shift towards integrating IV fluid monitoring devices with electronic health records (EHR) systems, allowing for better data management and analysis in clinical settings. This integration helps healthcare providers track patient progress efficiently and identify any complications in real time, improving clinical decision-making.
Additionally, the rise of telemedicine in the US has created a potential for remote monitoring solutions, enabling healthcare professionals to manage IV fluid therapy effectively without being physically present. These trends illustrate a transformative phase in the US IV Fluid Monitoring Devices Market, focusing on enhancing patient care through technology.
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
The rising healthcare expenditures in the United States significantly drive the growth of the US IV Fluid Monitoring Devices Market Industry. According to the Centers for Medicare & Medicaid Services (CMS), healthcare spending in the US was projected to increase from USD 3.6 trillion in 2018 to USD 6.2 trillion by 2028, indicating a compound annual growth rate of around 5.4%. Increased financial resources allow for the acquisition of advanced medical technologies, including IV fluid monitoring devices, thereby improving patient safety and care efficiency.
Established organizations like the American Hospital Association advocate for better healthcare funding, which has led to a greater focus on implementing sophisticated monitoring systems to enhance the quality of care, ultimately boosting the market for IV fluid monitoring devices.
The increasing prevalence of chronic diseases in the United States is a significant driver for the US IV Fluid Monitoring Devices Market Industry. Reports from the Centers for Disease Control and Prevention (CDC) highlight that approximately 6 in 10 adults in the US have a chronic disease, with conditions such as diabetes and heart disease requiring specialized monitoring and treatment. This surge in chronic diseases necessitates advanced medical solutions like IV fluid monitoring devices that help healthcare providers manage treatment effectively.
Key players in the market, such as Baxter International and Fresenius Kabi, are developing innovative IV fluid monitoring systems catering to chronic care management, enhancing the market's growth outlook.
Advancements in technology are propelling the growth of the US IV Fluid Monitoring Devices Market Industry. Innovations in medical devices, especially those that integrate digital health solutions, have transformed patient monitoring. The Journal of the American Medical Association reported that technologies such as mobile health applications and electronic health records have been widely adopted in the healthcare system, improving the tracking and management of IV fluids.
Leading manufacturers, such as B. Braun and Medtronic, are focusing on Research and Development to enhance device functionalities, contributing to market expansion and improving patient outcomes.
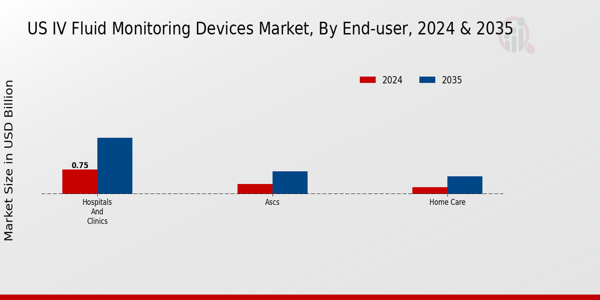
The End-user segment of the US IV Fluid Monitoring Devices Market plays a significant role in driving the overall market dynamics. This segment is primarily classified into Hospitals and clinics, Ambulatory Surgical Centers (ASCs), and Home care settings, each with its unique characteristics and requirements. Hospitals and clinics are pivotal in the landscape, given that they are often the primary point of care for patients requiring intravenous therapy, including surgeries and critical care situations.
The sophistication of technology used in these environments necessitates advanced IV fluid monitoring devices, ensuring patient safety and reducing complications. The growth in this area is propelled by increasing hospitalization rates and a higher prevalence of chronic diseases that require continuous monitoring. ASCs represent a growing segment as they offer outpatient surgical procedures that may require IV fluid management, thus providing a more cost-effective alternative compared to traditional hospital stays.
This shift towards outpatient care facilitates quicker recovery for patients and optimizes healthcare resource utilization, reflecting changing trends in patient care preferences in the US healthcare landscape. Home care proves to be an increasingly important segment, particularly for patients with chronic conditions, as it aligns with the rising trend of patient-centered care and emphasizes comfort and convenience.
The growing aging population in the US, along with advancements in technology allowing for safe monitoring in the home, is crucial to driving demand in this area. These diverse segments underline the broad applicability of IV Fluid Monitoring Devices throughout the continuum of care, emphasizing the importance of ongoing innovation and development to meet specific end-user needs effectively. Overall, the End-user segment reflects significant growth potential, driven by healthcare trends focusing on improved patient outcomes, efficiency, and the increasing adoption of advanced monitoring technologies across all settings in the US.
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
The US IV Fluid Monitoring Devices Market encompasses various types, primarily categorized into Desktop and Portable devices, which play essential roles in healthcare settings. The Desktop IV fluid monitoring devices offer extensive functionalities and are often used in critical care, providing reliable monitoring solutions for patients. They are significant in hospitals and clinics, managing large volumes of fluid administration.
Conversely, Portable IV fluid monitoring devices have gained traction due to their compact design and mobility, allowing healthcare professionals to monitor patients in diverse environments, including home healthcare and outpatient settings. This trend reflects the increasing demand for flexible healthcare solutions that promote patient safety and efficiency in fluid management. The growth in the US IV Fluid Monitoring Devices Market is further supported by advancements in technology, enhancing device capabilities and accuracy.
Additionally, the aging population and the rising prevalence of chronic diseases contribute to the increasing demand for effective fluid monitoring solutions. In summary, both Desktop and Portable types are key drivers of the US IV Fluid Monitoring Devices Market, showcasing unique advantages suited to various healthcare needs and settings.
The US IV Fluid Monitoring Devices Market is characterized by a dynamic competitive landscape wherein various firms compete to provide innovative solutions that enhance patient safety and improve clinical outcomes in healthcare settings. As the healthcare industry increasingly emphasizes patient-centric care and efficiency, the demand for advanced fluid monitoring devices continues to grow. This market includes a range of products such as infusion pumps and monitoring systems that ensure accurate drug delivery while reducing the likelihood of errors.
Companies in this sector must navigate regulatory challenges, integrate technological advancements, and respond to evolving customer needs to maintain their competitive edge. Collaborative efforts, strategic partnerships, and investments in research and development are crucial for companies aiming to establish a strong presence in this thriving market.
C.R. Bard is a notable player in the US IV Fluid Monitoring Devices Market, recognized for its innovation and high-quality products. The company's strengths lie in its comprehensive portfolio of IV access devices, along with a commitment to enhancing patient care through reliable and efficient products. With a strong focus on technology, C.R. Bard continually improves its offerings, which include advanced catheter systems and integrated monitoring solutions.
The company's established reputation and robust distribution network enable it to maintain a significant market presence, providing healthcare providers with trusted solutions that enhance overall treatment effectiveness. Through ongoing innovation and strategic investments, C.R. Bard is well positioned to meet the growing demands of the US healthcare market, solidifying its leading role in the IV fluid monitoring space.
Smiths Medical, another key player in the US IV Fluid Monitoring Devices Market, offers a diverse array of products and services aimed at improving patient outcomes in critical care environments. The company's portfolio includes infusion pumps, vascular access devices, and safety products designed to minimize risks associated with intravenous therapy. Smiths Medical’s strengths lie in its emphasis on both product quality and technological advancement, which fosters trust among healthcare practitioners.
The company has engaged in strategic mergers and acquisitions that have bolstered its market position and expanded its product lines, thereby enhancing its competitiveness in the US market. Smiths Medical’s ongoing commitment to innovation, customer service, and partnership-building further strengthens its footprint in the IV fluid monitoring sector, allowing it to effectively address the needs of healthcare facilities and ultimately improve patient care in the US.
Recent developments in the US IV Fluid Monitoring Devices Market have showcased significant advancements and activities. In August 2023, Baxter International launched a new IV fluid management system aimed at enhancing patient safety and improving clinical workflow. In the same month, Medtronic unveiled its latest infusion pump technology focused on precision dosing for critical care. The current market dynamics indicate a growing emphasis on technological enhancements and patient monitoring capabilities, reflecting a robust interest in improving healthcare outcomes.
In terms of mergers and acquisitions, in February 2023, ICU Medical announced its acquisition of the infusion and respiratory product lines from Smiths Medical, marking a strategic step to broaden its portfolio. Several companies have reported growth in market valuation, with Fresenius Kabi and Becton Dickinson seeing significant increases due to heightened demand for their advanced IV fluid solutions. Over the past few years, the market has been shaped by increased regulatory scrutiny and a surge in hospitals adopting smart technology, particularly as noted in policy shifts in 2021 aimed at improving patient care standards.
These factors continue to influence the overall landscape of the US IV Fluid Monitoring Devices Market.
| Report Attribute/Metric Source: | Details |
| MARKET SIZE 2018 | 1.1(USD Billion) |
| MARKET SIZE 2024 | 1.25(USD Billion) |
| MARKET SIZE 2035 | 3.0(USD Billion) |
| COMPOUND ANNUAL GROWTH RATE (CAGR) | 8.284% (2025 - 2035) |
| REPORT COVERAGE | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| BASE YEAR | 2024 |
| MARKET FORECAST PERIOD | 2025 - 2035 |
| HISTORICAL DATA | 2019 - 2024 |
| MARKET FORECAST UNITS | USD Billion |
| KEY COMPANIES PROFILED | C.R. Bard, Smiths Medical, GE Healthcare, HewlettPackard, Medtronic, Abbott Laboratories, Zynex Medical, Baxter International, Fresenius Kabi, Terumo, Vyaire Medical, ICU Medical, Halyard Health, Becton Dickinson, Philips Healthcare |
| SEGMENTS COVERED | End-user, Type |
| KEY MARKET OPPORTUNITIES | Telehealth integration for remote monitoring, Advanced data analytics for precision dosing, Increasing demand in outpatient settings, Growing focus on patient safety, Rising prevalence of chronic diseases |
| KEY MARKET DYNAMICS | growing demand for precision monitoring, increasing prevalence of chronic diseases, advancements in wearable technology, rising healthcare expenditure, emphasis on patient safety and outcomes |
| COUNTRIES COVERED | US |
Frequently Asked Questions (FAQ):
The US IV Fluid Monitoring Devices Market is expected to be valued at 1.25 billion USD in 2024.
By 2035, the market is projected to reach a value of 3.0 billion USD.
The US IV Fluid Monitoring Devices Market is expected to have a CAGR of 8.284% during the forecast period of 2025 to 2035.
By 2035, the Hospitals and clinics segment is forecasted to dominate the market with a value of 1.75 billion USD.
In 2024, the market revenue for ASCs is expected to be 0.3 billion USD, rising to 0.7 billion USD by 2035.
The Home care segment is expected to have a market value of 0.2 billion USD in 2024.
Major players in the market include C.R. Bard, Smiths Medical, GE Healthcare, and Medtronic, among others.
The Home care segment is projected to increase to 0.55 billion USD by 2035.
Emerging trends in telehealth and home-based therapies present significant growth opportunities in the market.
The overall growth rate is supported by technological advancements and an increasing demand for efficient healthcare solutions.
Leading companies partner with us for data-driven Insights.
Kindly complete the form below to receive a free sample of this Report
© 2025 Market Research Future ® (Part of WantStats Reasearch And Media Pvt. Ltd.)