US PD L1 Inhibitors Market Overview
As per MRFR analysis, the U.S. PD L1 Inhibitors Market Size was estimated at 2.77 (USD Billion) in 2024.The U.S. PD L1 Inhibitors Market Industry is expected to grow from 2.98(USD Billion) in 2025 to 7.17 (USD Billion) by 2035. The U.S. PD L1 Inhibitors Market CAGR (growth rate) is expected to be around 8.294% during the forecast period (2025 - 2035).
Key U.S. PD L1 Inhibitors Market Trends Highlighted
The U.S. PD L1 Inhibitors Market is seeing several key drivers that are shaping its future. The increasing prevalence of cancer, particularly lung cancer, has spurred demand for effective immunotherapy treatments. Health care reforms and the expansion of health insurance coverage have improved access to these therapies, allowing more patients to receive PD L1 inhibitors. Additionally, ongoing advancements in biotechnology play a significant role, as pharmaceutical companies invest in research and development to create new and more effective product formulations. Recent trends reflect a growing trend toward personalized medicine, where treatment plans are tailored to individual patient profiles.
This approach is facilitated by advancements in biomarker testing, which help in identifying suitable candidates for PD L1 therapy. Moreover, there are opportunities to be explored in the areas of combination therapies, where PD L1 inhibitors are used alongside other treatment modalities to enhance efficacy and patient outcomes. Collaborations between academic institutions and pharmaceutical firms in the U.S. are increasingly fostering innovation in this drug class, potentially leading to new clinical applications and combinations that could benefit patients.
The increase in clinical trials focusing on diverse patient populations is another emerging trend, aiming to ensure that a wider demographic can benefit from these therapies. This approach is vital in improving health equity and addressing disparities in cancer treatment across the U.S. Overall, as research continues and regulations adapt, the PD L1 inhibitors market is poised for continuous growth driven by these dynamic trends in the United States.
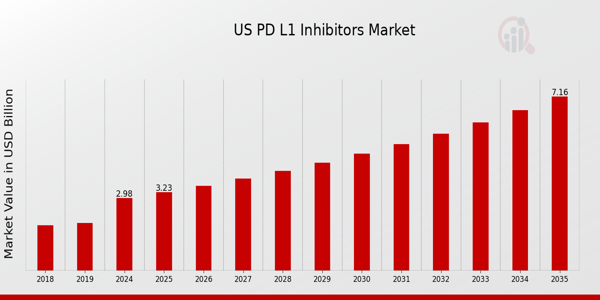
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
U.S. PD L1 Inhibitors Market Drivers
Increasing Incidence of Cancer
The rising incidence of various cancers in the United States is significantly driving the growth of the U.S. PD L1 Inhibitors Market Industry. According to the American Cancer Society, an estimated 1.9 million new cancer cases were expected to be diagnosed in the U.S. in 2021, with projections suggesting that this number will continue to rise. This trend underscores a growing patient population in need of advanced therapies such as PD L1 inhibitors. Advanced immunotherapy treatments that block programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have shown promising results in clinical studies.
Institutions like the National Cancer Institute are promoting PD L1 inhibitor research and development, which will probably be beneficial to the market. The rising number of patients with diagnoses looking for treatment will likely increase the need for PD L1 inhibitors, significantly increasing revenue in the U.S. PD L1 Inhibitors Market.
Advancements in Immunotherapy Research
Recent advancements in immunotherapy are propelling the U.S. PD L1 Inhibitors Market Industry forward. The increase in funding for cancer research and numerous clinical trials has accelerated the development of PD L1 inhibitors, making them a lucrative treatment option. The National Institutes of Health reported a rise in federal funding for cancer research, which was approximately 6.5 billion USD in 2020. Such developments enhance the understanding and efficacy of PD L1 inhibitors. Furthermore, pharmaceutical companies like Merck and Bristol Myers Squibb continue to invest heavily in Research and Development, resulting in new therapies and treatment combinations entering the market.
These innovations not only expand the existing product portfolio but also optimize treatment regimens, making these therapies more appealing to oncologists and patients alike.
Regulatory Support for Novel Treatments
The regulatory landscape in the U.S. is increasingly favorable for novel cancer treatments, shaping the growth of the U.S. PD L1 Inhibitors Market Industry. The Food and Drug Administration has implemented various expedited approval pathways, such as Breakthrough Therapy Designation and Accelerated Approval, to fast-track life-saving drugs. In 2021, the FDA granted approval to multiple PD L1 inhibitors, based on their ability to significantly improve patient outcomes in clinical trials. These regulatory efforts encourage pharmaceutical companies to invest Resources into developing innovative treatments, leading to an expanded market presence for PD L1 inhibitors.
Furthermore, the increased focus on patient-centric treatment options aligns with the broader healthcare trend towards personalized medicine, further solidifying the position of PD L1 inhibitors within the oncology paradigm.
U.S. PD L1 Inhibitors Market Segment Insights
PD L1 Inhibitors Market Type Insights
The U.S. PD L1 Inhibitors Market has garnered significant attention due to its classification into various Types, thereby presenting a comprehensive landscape for understanding market dynamics and growth opportunities. The market is categorized into Monoclonal Antibodies, Small Molecule Inhibitors, and Combination Therapy, each presenting unique advantages and challenges that drive their development and application. Monoclonal Antibodies have established themselves as critical players in immunotherapy by targeting PD-L1 proteins effectively, fostering an enhanced immune response against tumors.
This class has witnessed considerable advancements, showcasing high specificity, which translates to improved efficacy and reduced side effects in patients, thus making them highly relevant in the treatment of various cancers.
On the other hand, small-molecule inhibitors provide a different mechanism of action, typically focusing on intracellular pathways for PD-L1 modulation. This type has emerged as a promising alternative due to its potential for oral administration and better tissue penetration, which can lead to easier patient compliance and convenience. The design of these inhibitors often aims to complement the effects of Monoclonal Antibodies, providing a multidimensional approach to cancer treatment, which can be beneficial in overcoming the resistance sometimes exhibited by tumors against single-agent therapies.
Combination Therapy is increasingly recognized for its potential to enhance treatment outcomes by using multiple modalities to target cancer from various angles simultaneously.
In the context of PD L1 Inhibitors, combining different types of inhibitors or integrating them with other therapeutic approaches, such as chemotherapy or radiation therapy, has shown promise in amplifying anti-tumor activity and improving patient prognoses. This trend is supported by numerous clinical studies that highlight the synergistic benefits of combining therapies, thus driving interest and investment in this segment.
Overall, the interplay between these types within the U.S. PD L1 Inhibitors Market is crucial, as they collectively contribute to the evolving landscape of cancer treatment. Market drivers like stricter regulatory approvals, ongoing clinical trials, and increasing governmental initiatives aimed at fostering innovation play key roles in propelling growth in this domain. Furthermore, the established infrastructure for drug development and Research and Development in the U.S. provides a conducive environment for these therapies to flourish. The continuous demand for effective cancer treatments also opens numerous opportunities for investment and research, making the U.S.
PD L1 Inhibitors Market a focal point for drug developers and researchers alike. As new data emerges, the adaptability of these segments to integrate advanced biotechnological methods presents a strong foundation for future advancements in cancer therapies.
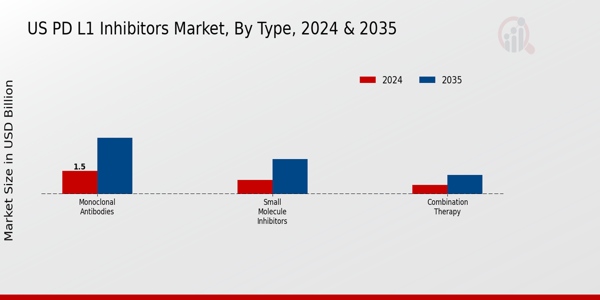
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
PD L1 Inhibitors Market Indication Insights
The U.S. PD L1 Inhibitors Market is witnessing notable growth in the Indication segment, primarily driven by the rising incidence of various cancers. Non-Small Cell Lung Cancer represents a significant share, as it accounts for a large percentage of lung cancer diagnoses in the United States, with advancements in therapies contributing to improved patient outcomes. Additionally, Breast Cancer continues to be a major area of focus due to its prevalence among women, prompting extensive Research and Development efforts to enhance treatment options.
Bladder Cancer also holds importance in the market as current therapies often lack effectiveness, leading to a growing need for innovative solutions. Hepatocellular Carcinoma is gaining attention due to the increasing number of liver cancer cases, often linked to chronic liver diseases, presenting opportunities for PD L1 inhibitors to play a pivotal role in treatment strategies. Melanoma, known for its aggressive nature, remains a critical area within the market as immunotherapy advances; it commands significant research interest due to its rising incidence rates. Overall, the U.S.
PD L1 Inhibitors Market segmentation highlights diverse opportunities and potential benefits that these therapies bring to patients across various cancer types.
PD L1 Inhibitors Market Administration Route Insights
The Administration Route segment of the U.S. PD L1 Inhibitors Market plays a crucial role in the delivery of immunotherapy agents, significantly impacting patient outcomes and market dynamics. This segment encompasses various methods such as Intravenous, Subcutaneous, and Oral administration, each presenting unique advantages and challenges. Intravenous administration is widely recognized for its rapid onset of action and effective delivery of high doses, often leading to improved therapeutic outcomes for patients with advanced cancers.
In contrast, Subcutaneous administration offers benefits in terms of patient convenience and ease of use, allowing for at-home administration, which is increasingly important for patient compliance and comfort. Furthermore, the Oral route represents a growing trend, providing a non-invasive and easily acceptable option for many patients, potentially enhancing treatment adherence. As the U.S. healthcare market increasingly emphasizes patient-centered care, these diverse administration routes reflect the evolving landscape of cancer care, contributing to the overall growth and development of the U.S. PD L1 Inhibitors Market.
With patients actively seeking personalized treatment options, the market continues to adapt, driven by innovations in drug formulation and administration technologies, responding to both the clinical needs and preferences of patients.
PD L1 Inhibitors Market End User Insights
The end-user segment of the U.S. PD L1 Inhibitors Market plays a vital role in the overall dynamics of the industry, catering primarily to Hospitals, Oncology Clinics, and Research Laboratories. Hospitals are a significant component, as they facilitate comprehensive care and treatment for cancer patients, integrating PD L1 inhibitors into various therapeutic protocols. Oncology Clinics focus on specialized cancer treatments and have seen a marked increase in the administration of these inhibitors, driven by ongoing clinical advancements and patient demand for targeted therapies.
Research Laboratories support the advancement of the field through rigorous studies and trials, enhancing the understanding and application of PD L1 inhibitors. This segment is pivotal in the market, reflecting the growing focus on personalized medicine and the pressing need for effective cancer treatment solutions in the U.S. Factors such as rising incidence rates of cancer and ongoing innovations in immunotherapy are propelling market growth, while challenges like reimbursement issues and varying adoption rates across regions play a significant role in shaping the landscape.
With continued investment and research efforts, the end-user segment is poised to evolve, contributing to advancements and a broader application of PD L1 inhibitors in patient care.
U.S. PD L1 Inhibitors Market Key Players and Competitive Insights
The U.S. PD L1 Inhibitors Market has become increasingly competitive, showcasing a dynamic environment driven by rapid advancements in cancer immunotherapy. As the demand for effective oncology treatments grows, numerous pharmaceutical companies are vying for dominance in this lucrative market. A multitude of companies are focusing on the research and development of PD L1 inhibitors to boost their pipelines and cater to the increasing needs of patients with various types of cancers, including non-small cell lung cancer and other solid tumors.
This competitive landscape is characterized by innovative product offerings, strategic collaborations, and extensive clinical trials aimed at gaining a significant share in this evolving sector. Companies are not only striving to enhance their therapeutic options but also aiming to expand their market presence through robust marketing strategies and partnerships with healthcare providers. Celgene, a key player in the U.S. PD L1 Inhibitors Market, leverages its strong research capabilities and established reputation to capitalize on opportunities within this segment.
The company has garnered significant support from healthcare professionals and stakeholders due to its innovative approach to drug development and commitment to patient-centered care. Celgene's strengths lie in its extensive clinical trial experience and robust pipeline, allowing it to stay ahead of the competition. The company's strategic partnerships further enhance its position, enabling it to amplify its market reach and strengthen its therapeutic offerings in the PD L1 domain. Celgene's ongoing focus on developing next-generation inhibitors positions it favorably to address the escalating demand for cancer treatments in the U.S. market.
Gilead Sciences maintains a strong market presence in the U.S. PD L1 Inhibitors Market through its innovative product portfolio designed to meet the therapies required for diverse cancer patients. Known for its commitment to research and development, Gilead has successfully introduced key PD L1 inhibitors that enhance treatment efficacy and patient outcomes. The company’s strengths include its extensive expertise in oncology and its well-established relationships with healthcare providers and research institutions, facilitating the swift adoption of its therapies. Gilead Sciences has also engaged in strategic mergers and acquisitions, allowing it to diversify its offerings and bolster its competitive edge.
Through these initiatives, Gilead continues to reinforce its commitment to improving cancer care in the U.S., driving essential advancements in the evolving landscape of PD L1 inhibitors.
Key Companies in the U.S. PD L1 Inhibitors Market Include:
- Regeneron Pharmaceuticals
U.S. PD L1 Inhibitors Market Industry Developments
The U.S. PD L1 Inhibitors Market has seen significant advancements recently, particularly with the ongoing research and development efforts by companies such as Merck and Bristol Myers Squibb, focusing on innovative immune-oncology therapies. In March 2023, Gilead Sciences announced an agreement to acquire a biopharmaceutical company specializing in PD L1 therapies, which enhances its portfolio in the oncology sector.
Meanwhile, Regeneron Pharmaceuticals is progressing with clinical trials for its PD L1 inhibitors, demonstrating promising results in combination with other therapies. The market valuation of companies like Roche and AstraZeneca has markedly increased, contributing to heightened competition and innovation within the sector.
Additionally, the rise in demand for personalized medicine and immunotherapies has been bolstering market dynamics over the past two years, reflecting a robust growth trajectory in oncology treatments. Notably, collaborations between Pfizer and Eli Lilly for research and clinical trials signify a trend of partnerships aimed at expediting drug development in this critical area of cancer therapy.
Pd L1 Inhibitors Market Segmentation Insights
PD L1 Inhibitors Market Type Outlook
- Small Molecule Inhibitors
PD L1 Inhibitors Market Indication Outlook
- Non-Small Cell Lung Cancer
PD L1 Inhibitors Market Administration Route Outlook
PD L1 Inhibitors Market End User Outlook
|
Report Attribute/Metric
|
Details
|
|
Market Size 2024
|
2.77(USD Billion)
|
|
Market Size 2025
|
2.98(USD Billion)
|
|
Market Size 2035
|
7.17(USD Billion)
|
|
Compound Annual Growth Rate (CAGR)
|
8.294% (2025 - 2035)
|
|
Report Coverage
|
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends
|
|
Base Year
|
2024
|
|
Market Forecast Period
|
2025 - 2035
|
|
Historical Data
|
2019 - 2024
|
|
Market Forecast Units
|
USD Billion
|
|
Key Companies Profiled
|
Celgene, Gilead Sciences, Sanofi, Regeneron Pharmaceuticals, Merck & Co, Pfizer, Bristol Myers Squibb, AstraZeneca, Incyte Corporation, Novartis, Roche, Eli Lilly, Amgen, Boehringer Ingelheim
|
|
Segments Covered
|
Type, Indication, Administration Route, End User
|
|
Key Market Opportunities
|
Rising cancer prevalence,
Expanding immunotherapy applications, Increased R&D investments,
Emerging combination therapies,
Growing awareness and education
|
|
Key Market Dynamics
|
Rising cancer prevalence,
Innovative drug development,
Competitive pricing strategies,
Regulatory approvals,
Expanding patient access
|
|
Countries Covered
|
U.S.
|
Frequently Asked Questions (FAQ):
The U.S. PD L1 Inhibitors Market was expected to be valued at 2.98 billion USD in 2024.
By 2035, the U.S. PD L1 Inhibitors Market is projected to reach a value of 7.17 billion USD.
The expected CAGR for the U.S. PD L1 Inhibitors Market during the forecast period is 8.294 percent.
Key players in the U.S. PD L1 Inhibitors Market include companies like Merck & Co, Bristol Myers Squibb, AstraZeneca, and Roche.
The Monoclonal Antibodies segment is expected to dominate with a market size of 3.66 billion USD by 2035.
The Small Molecule Inhibitors segment is anticipated to reach 2.27 billion USD in market size by 2035.
The Combination Therapy segment is projected to have a market value of 1.24 billion USD by 2035.
The market growth across segments is driven by increasing applications in cancer treatment and advancements in immunotherapy.
Emerging opportunities include innovative treatment approaches and collaborations between key market players.
The market might face challenges such as regulatory hurdles and competition from alternative therapies.
















