Advancements in Treatment Modalities
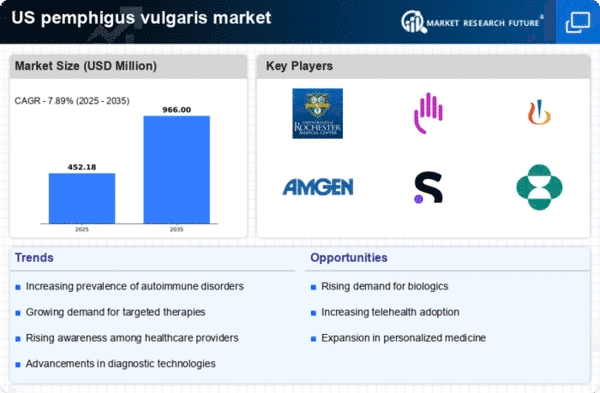
Innovations in treatment modalities are significantly impacting the pemphigus vulgaris market. The introduction of novel therapies, including monoclonal antibodies and targeted therapies, has transformed the management of this condition. For instance, therapies such as rituximab have shown promising results in clinical trials, leading to increased adoption among healthcare providers. The market is projected to reach approximately $1 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 8%. These advancements not only improve patient outcomes but also expand the treatment landscape, making it essential for stakeholders in the pemphigus vulgaris market to stay abreast of emerging therapies and their clinical efficacy.
Rising Incidence of Pemphigus Vulgaris
The pemphigus vulgaris market is experiencing growth due to the increasing incidence of this autoimmune disorder in the US. Recent estimates suggest that the prevalence of pemphigus vulgaris is approximately 0.5 to 3 cases per 100,000 individuals. Recent estimates suggest that the prevalence of pemphigus vulgaris is approximately 0.5 to 3 cases per 100,000 individuals, indicating a rising trend. This increase in cases necessitates the development of effective treatment options, thereby driving demand within the pemphigus vulgaris market. Furthermore, as awareness of the disease grows among healthcare professionals and patients, early diagnosis and intervention become more common, potentially leading to improved patient outcomes. The rising incidence not only highlights the need for innovative therapies but also emphasizes the importance of ongoing research and development efforts in the pemphigus vulgaris market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is playing a pivotal role in shaping the pemphigus vulgaris market. The US Food and Drug Administration (FDA) has implemented various initiatives to expedite the approval process for new treatments, particularly those addressing rare diseases like pemphigus vulgaris. This regulatory environment encourages pharmaceutical companies to invest in the development of novel therapies, which may lead to a broader range of treatment options for patients. As a result, the pemphigus vulgaris market is likely to see an influx of new products, enhancing competition and potentially lowering treatment costs, ultimately benefiting patients.
Increased Awareness and Education Initiatives
The pemphigus vulgaris market is benefiting from heightened awareness and education initiatives aimed at both healthcare professionals and patients. Organizations dedicated to autoimmune diseases are actively promoting educational campaigns to inform stakeholders about the symptoms, diagnosis, and treatment options available for pemphigus vulgaris. This increased awareness is likely to lead to earlier diagnosis and treatment, which can improve patient outcomes. Furthermore, as more healthcare providers become knowledgeable about the condition, the demand for effective therapies within the pemphigus vulgaris market is expected to rise. This trend underscores the importance of continuous education in driving market growth.
Growing Investment in Research and Development
Investment in research and development (R&D) is a crucial driver for the pemphigus vulgaris market. Pharmaceutical companies and research institutions are increasingly allocating resources to explore new therapeutic options and improve existing treatments. This trend is evidenced by the rising number of clinical trials focused on pemphigus vulgaris, with over 50 ongoing studies in the US alone. Such investments are likely to yield innovative solutions that address unmet medical needs, thereby enhancing the pemphigus vulgaris market. Additionally, collaborations between academia and industry are fostering a conducive environment for breakthroughs in treatment, which may lead to more effective management strategies for patients.
















Leave a Comment