Growing Awareness of Skin Health
The plastic surgery-integumentary-system market is influenced by a growing awareness of skin health and its importance in overall well-being. Consumers are increasingly educated about the effects of aging, sun exposure, and lifestyle choices on skin quality. This awareness drives demand for procedures such as facelifts, chemical peels, and skin rejuvenation treatments. The market is expected to see a rise in the adoption of preventive measures, with individuals seeking to maintain youthful appearances through both surgical and non-surgical options. Furthermore, the integration of dermatology and plastic surgery practices is likely to enhance patient outcomes, as practitioners can offer comprehensive care tailored to individual needs. This trend suggests a potential shift in consumer behavior, with a focus on long-term skin health rather than solely aesthetic improvements.
Advancements in Surgical Techniques
Innovations in surgical techniques significantly impact the plastic surgery-integumentary-system market. Minimally invasive procedures, such as endoscopic techniques and laser-assisted surgeries, have gained traction due to their reduced recovery times and lower complication rates. These advancements not only enhance patient safety but also improve overall satisfaction, leading to increased patient referrals. The market has seen a shift towards outpatient procedures, which are often more cost-effective and convenient for patients. As a result, the plastic surgery-integumentary-system market is likely to expand as more individuals opt for these advanced techniques. Furthermore, the integration of 3D imaging and virtual reality in pre-operative planning is revolutionizing the way surgeons approach procedures, potentially increasing the precision and outcomes of surgeries.
Influence of Health and Wellness Trends
The plastic surgery-integumentary-system market is increasingly shaped by the broader health and wellness trends that emphasize holistic well-being. As consumers become more health-conscious, there is a growing interest in procedures that not only enhance appearance but also promote physical health. For instance, body contouring procedures are often sought after by individuals looking to improve their fitness results. This trend is supported by a rise in fitness culture and the popularity of wellness programs, which encourage individuals to invest in their bodies. The market may see a potential increase in demand for procedures that align with these health-oriented goals, suggesting a shift in consumer priorities. As a result, practitioners are likely to adapt their services to cater to this evolving mindset, further driving growth in the plastic surgery-integumentary-system market.
Rising Demand for Aesthetic Enhancements
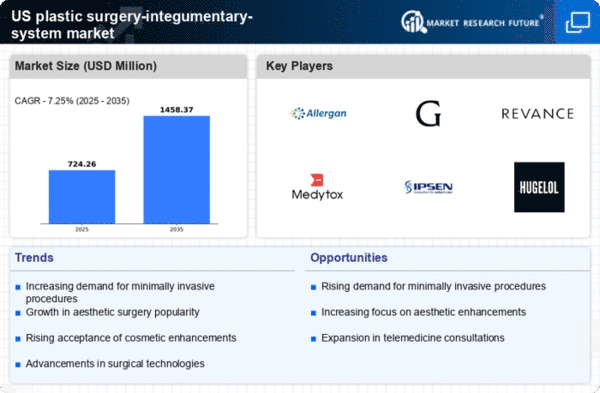
The plastic surgery-integumentary-system market experiences a notable increase in demand for aesthetic enhancements. This trend is driven by a growing societal emphasis on physical appearance and self-image. According to recent data, the market is projected to reach approximately $20 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 8%. This rising demand is particularly evident among younger demographics, who are increasingly seeking procedures such as rhinoplasty and liposuction. The influence of celebrity culture and media representation further fuels this desire for aesthetic improvements, leading to a more competitive landscape among service providers. As a result, practitioners are adapting their offerings to meet the evolving preferences of consumers, thereby contributing to the overall growth of the plastic surgery-integumentary-system market.
Increased Accessibility to Cosmetic Procedures
The plastic surgery-integumentary-system market benefits from increased accessibility to cosmetic procedures. The proliferation of clinics and surgical centers across urban and suburban areas has made these services more available to a broader audience. Additionally, financing options and payment plans have emerged, allowing patients to afford procedures that were previously considered luxury services. This trend is particularly relevant in the context of the rising middle class, which is more willing to invest in personal appearance. As a result, the market is projected to grow, with estimates suggesting a potential increase of 10% in the number of procedures performed annually. This accessibility not only enhances consumer choice but also stimulates competition among providers, leading to improved service quality and innovation.