Rising Demand for Biologics
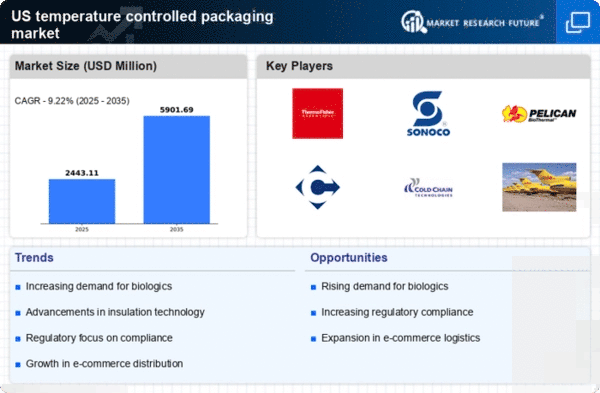
The increasing prevalence of chronic diseases and the aging population in the US are driving the demand for biologics, which require temperature controlled packaging for safe transportation. The temperature controlled-packaging-pharmaceutical market is experiencing growth as biologics often necessitate strict temperature regulations to maintain efficacy. According to industry reports, the biologics market is projected to reach approximately $500 billion by 2025, indicating a substantial opportunity for temperature controlled solutions. This trend underscores the importance of reliable packaging systems that can ensure the integrity of sensitive products during transit, thereby enhancing the overall value proposition of the temperature controlled-packaging-pharmaceutical market.
Increased Focus on Patient Safety
Patient safety remains a critical concern in the pharmaceutical industry, driving the demand for temperature controlled packaging solutions. The temperature controlled-packaging-pharmaceutical market is influenced by the need to prevent product spoilage and ensure that medications are delivered within specified temperature ranges. Regulatory bodies are increasingly emphasizing the importance of maintaining the cold chain, which is essential for the efficacy of many pharmaceuticals. This heightened focus on patient safety is likely to propel investments in advanced packaging technologies that can provide real-time monitoring and alerts, thereby enhancing the reliability of temperature controlled solutions.
Regulatory Pressures and Compliance
The temperature controlled packaging market is significantly influenced by regulatory pressures aimed at ensuring product safety and efficacy. Regulatory agencies in the US are enforcing stringent guidelines regarding the transportation and storage of temperature sensitive pharmaceuticals. Compliance with these regulations is essential for pharmaceutical companies to avoid penalties and ensure market access. As a result, there is a growing need for reliable temperature controlled packaging solutions that can meet these regulatory requirements. This trend is expected to drive investments in compliance-focused packaging technologies, thereby enhancing the overall growth prospects of the temperature controlled-packaging-pharmaceutical market.
Technological Innovations in Packaging
Technological advancements are playing a pivotal role in transforming the temperature controlled-packaging-pharmaceutical market. Innovations such as smart packaging, which incorporates IoT technology, allow for real-time monitoring of temperature and humidity levels during transit. These advancements not only enhance the safety and efficacy of temperature sensitive products but also improve operational efficiencies for pharmaceutical companies. The integration of data analytics and machine learning in packaging solutions is expected to streamline logistics and reduce costs, thereby fostering growth in the temperature controlled-packaging-pharmaceutical market. As companies seek to optimize their supply chains, the demand for such innovative solutions is likely to increase.
Expansion of E-commerce in Pharmaceuticals
The rapid growth of e-commerce in the pharmaceutical sector is reshaping the landscape of the temperature controlled-packaging-pharmaceutical market. As more consumers opt for online purchasing of medications, the need for effective temperature controlled solutions becomes paramount. E-commerce sales in the pharmaceutical industry are expected to grow at a CAGR of around 15% through 2025, necessitating innovative packaging solutions that can maintain product integrity during shipping. This shift not only increases the volume of temperature sensitive products being shipped but also emphasizes the need for robust tracking and monitoring systems within the temperature controlled-packaging-pharmaceutical market.