Rising Incidence of Heart Failure
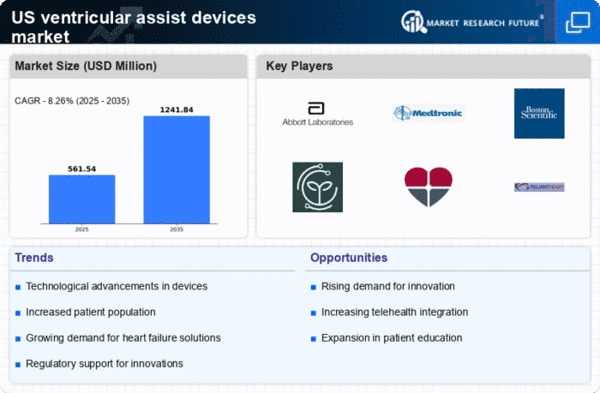
The increasing prevalence of heart failure in the US is a primary driver for the ventricular assist-devices market. According to the American Heart Association, approximately 6.2 million adults in the US are living with heart failure, a number that is projected to rise significantly. This growing patient population necessitates advanced treatment options, including ventricular assist devices, to manage symptoms and improve quality of life. As healthcare providers seek effective solutions to address this rising burden, the demand for ventricular assist-devices is likely to increase. Furthermore, the aging population, which is more susceptible to cardiovascular diseases, contributes to this trend. The ventricular assist-devices market is thus positioned for growth as it responds to the urgent need for innovative therapies to support heart failure patients.
Expansion of Reimbursement Policies
The expansion of reimbursement policies for ventricular assist devices is a critical driver for the market. Insurance providers are increasingly recognizing the value of these devices in improving patient outcomes and reducing long-term healthcare costs. As reimbursement coverage expands, more patients will have access to ventricular assist devices, which could lead to a surge in demand. The Centers for Medicare & Medicaid Services (CMS) has made strides in updating its policies to include these devices, reflecting a growing acknowledgment of their clinical benefits. This trend is likely to encourage healthcare providers to adopt ventricular assist devices more widely, thereby propelling the growth of the ventricular assist-devices market.
Technological Innovations in Device Design
Innovations in the design and functionality of ventricular assist devices are propelling the market forward. Recent advancements have led to the development of smaller, more efficient devices that offer improved patient outcomes. For instance, the introduction of continuous-flow devices has shown to enhance hemodynamic performance while reducing complications associated with traditional pulsatile devices. The ventricular assist-devices market is witnessing a shift towards miniaturization and biocompatibility, which not only improves patient comfort but also expands the potential for outpatient management. As these technologies evolve, they are likely to attract more patients and healthcare providers, thereby driving market growth. The investment in research and development by leading manufacturers further indicates a commitment to innovation in this sector.
Increased Funding for Cardiovascular Research
The ventricular assist-devices market is benefiting from increased funding for cardiovascular research in the US. Government initiatives and private sector investments are focusing on developing advanced therapies for heart failure and other cardiovascular conditions. For example, the National Institutes of Health (NIH) has allocated substantial resources towards research aimed at improving heart pump technologies. This financial support fosters innovation and accelerates the development of new devices, which is crucial for the ventricular assist-devices market. As more funding becomes available, it is expected that breakthroughs in device technology will emerge, enhancing treatment options for patients and potentially leading to a larger market share for these devices.
Growing Awareness and Education on Heart Health
There is a notable increase in awareness and education regarding heart health among the US population, which is positively impacting the ventricular assist-devices market. Public health campaigns and educational programs are emphasizing the importance of early detection and management of heart diseases. As individuals become more informed about heart failure and its treatment options, they are more likely to seek medical advice and explore advanced therapies, including ventricular assist devices. This heightened awareness is likely to lead to earlier diagnoses and increased demand for these devices. The ventricular assist-devices market stands to gain as patients actively pursue innovative solutions to manage their heart conditions.