Increased Focus on Real-World Evidence
The virtual clinical-trials market is benefiting from an increased focus on real-world evidence (RWE) in clinical research. Regulatory bodies, including the FDA, are encouraging the use of RWE to support drug development and approval processes. This trend is fostering a more favorable environment for virtual trials, as they can efficiently collect real-world data from diverse patient populations. The integration of RWE into clinical trials is expected to enhance the relevance and applicability of trial findings, thereby improving the overall quality of evidence generated. As the demand for RWE continues to grow, the virtual clinical-trials market is likely to expand, with sponsors increasingly adopting virtual methodologies to gather comprehensive data that reflects actual patient experiences.
Cost Efficiency and Resource Optimization
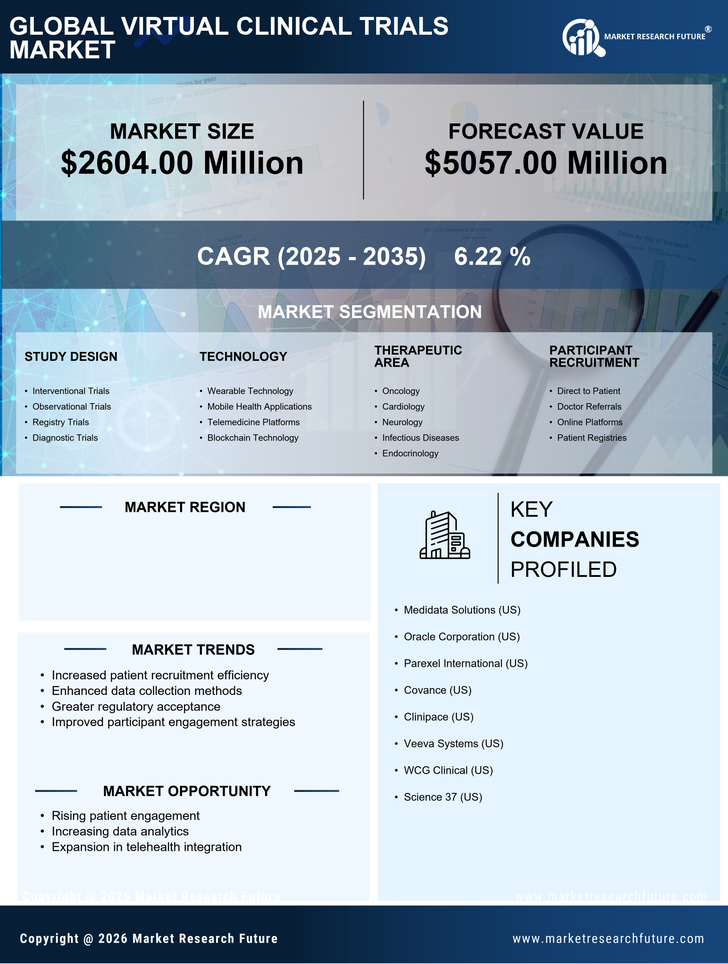
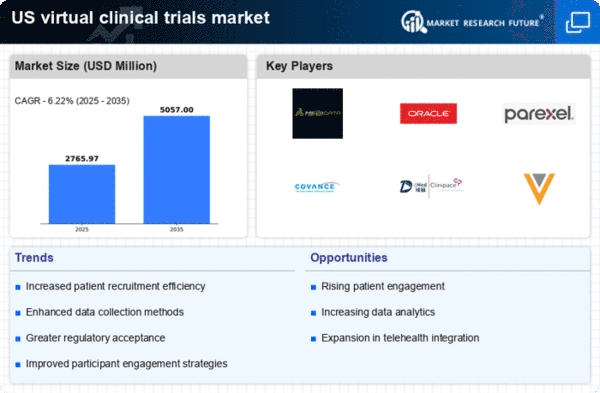
Cost efficiency is a critical driver for the virtual clinical-trials market, as organizations seek to reduce the financial burden associated with traditional clinical trials. Virtual trials can significantly lower operational costs by minimizing the need for physical sites and reducing patient recruitment expenses. Research indicates that virtual trials can save up to 30% in overall trial costs compared to conventional methods. This financial incentive is compelling for sponsors, particularly in an environment where the average cost of bringing a new drug to market exceeds $2.6 billion. By leveraging virtual methodologies, organizations can optimize resource allocation and enhance trial feasibility, making the virtual clinical-trials market an attractive option for stakeholders aiming to maximize return on investment.
Technological Advancements in Digital Health
The virtual clinical-trials market is experiencing a surge due to rapid technological advancements in digital health. Innovations such as wearable devices, mobile health applications, and remote monitoring tools are enhancing patient engagement and data collection. These technologies facilitate real-time data transmission, which is crucial for the efficiency of virtual trials. According to recent estimates, the digital health market in the US is projected to reach approximately $500 billion by 2025, indicating a robust growth trajectory. This growth is likely to drive the virtual clinical-trials market as sponsors increasingly leverage these technologies to streamline trial processes and improve patient outcomes. Furthermore, the integration of artificial intelligence and machine learning into trial design and execution is expected to optimize resource allocation and enhance predictive analytics, thereby fostering a more efficient virtual clinical-trials market.
Growing Demand for Patient-Centric Approaches
The virtual clinical-trials market is being propelled by a growing demand for patient-centric approaches in clinical research. Patients are increasingly seeking trials that accommodate their lifestyles, and virtual trials offer the flexibility and convenience that traditional trials often lack. This shift towards patient-centricity is reflected in the rising number of patients willing to participate in virtual trials, with studies indicating that up to 70% of patients prefer remote participation options. As a result, pharmaceutical companies and research organizations are adapting their strategies to meet these preferences, thereby expanding the virtual clinical-trials market. Additionally, the emphasis on diversity and inclusion in clinical research is prompting sponsors to utilize virtual trials to reach underrepresented populations, further enhancing the appeal and accessibility of clinical studies.
Regulatory Evolution Supporting Virtual Trials
The virtual clinical-trials market is witnessing a favorable regulatory evolution that supports the adoption of innovative trial designs. Regulatory agencies are increasingly recognizing the potential of virtual trials to enhance patient access and streamline the research process. Recent guidelines have been established to facilitate the use of digital tools and remote monitoring in clinical trials, thereby providing a clearer framework for sponsors. This regulatory support is crucial, as it encourages organizations to invest in virtual methodologies, knowing that they align with compliance requirements. As regulations continue to evolve, the virtual clinical-trials market is expected to grow, with more sponsors willing to explore these innovative approaches to clinical research.