Vaccine Contract Development and Manufacturing Organization Market Overview
The Vaccine Contract Development and Manufacturing Organization Market Size was estimated at 4.24 (USD Billion) in 2023.The Vaccine Contract Development and Manufacturing Organization Market is expected to grow from 4.63(USD Billion) in 2024 to 12.0 (USD Billion) by 2035. The Vaccine Contract Development and Manufacturing Organization Market CAGR (growth rate) is expected to be around 9.05% during the forecast period (2025 - 2035).
Key Vaccine Contract Development and Manufacturing Organization Market Trends Highlighted
The Global Vaccine Contract Development and Manufacturing Organization (CDMO) market is witnessing significant growth driven by increasing vaccine demand and the need for advanced manufacturing capabilities. The urgency for the rapid development of vaccines, especially in light of recent global health challenges, has intensified the focus on outsourcing manufacturing processes. Pharmaceutical companies are realizing the advantages of partnering with CDMOs to enhance efficiency and reduce time-to-market for new vaccines, leading to expanded collaboration within the pharmaceutical ecosystem. Moreover, the push for innovative vaccine technologies, such as mRNA-based therapies, is further propelling the need for specialized manufacturing services.
There are numerous opportunities to be explored within the vaccine CDMO landscape. The rise of emerging markets presents a chance for companies to expand their footprint in regions with increasing healthcare needs. Additionally, advancements in biomanufacturing techniques and automation offer pathways to enhance production efficiency. Companies that can adapt to new technologies and provide customized solutions may capitalize on the growing need for diverse vaccine platforms. Collaborations with biotech firms can also offer pathways to leverage cutting-edge research and development capabilities, potentially resulting in new vaccine formulations that address unmet medical needs.
Recent trends indicate a shift towards more flexible and scalable production processes, with CDMOs investing in modern facilities and technologies to meet the diverse requirements of vaccine development. The integration of digital technologies into manufacturing processes is becoming more common, allowing for improved quality control and data management. Sustainability is another key concern, leading to an increased focus on environmentally friendly practices within vaccine production. As regulatory frameworks evolve, CDMOs are adapting to ensure compliance while maintaining high standards of safety and efficacy in vaccine manufacturing.
Overall, the dynamic nature of this market underscores the importance of strategic partnerships and innovation in navigating the complexities of vaccine development and supply.
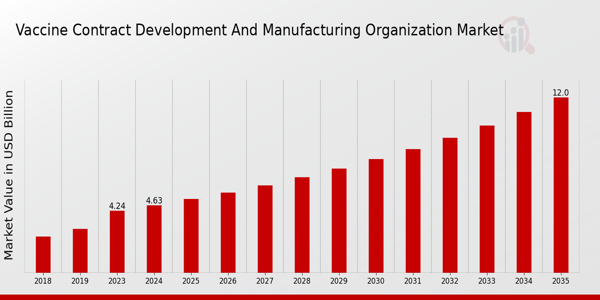
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
Vaccine Contract Development and Manufacturing Organization Market Drivers
Increasing Demand for Vaccines due to Global Health Threats
The Vaccine Contract Development and Manufacturing Organization Market is growing at a remarkable rate because of the sustained need for vaccines after a global pandemic or any new infectious disease occurs. Events like the COVID pandemic have highlighted the need for faster vaccine deployment, resulting in heightened urgency in vaccine development. The constantly increasing awareness regarding vaccination in order to safeguard public health is leading to an increased demand for vaccines.
All of this is combined with the need for improved infrastructure in the development and manufacture of the vaccine, resulting in the boom of the Vaccine Contract Development and Manufacturing Organization Market. Biopharma innovation continues through the application of new manufacturing technologies, which subsequently improve the turnaround time for vaccine production. There is also a movement towards improved vaccine reach in neglected areas, which calls for the cooperation of stakeholders, government, NGOs and private sectors.
The public health focus, coupled with the ability to respond to critical healthcare needs, is enhancing the market scenario for contract development and manufacturing organizations. Overall, this new global vaccination race has created new business opportunities for organizations participating in the Vaccine Contract Development and Manufacturing Organization Market and places them in a very advantageous position for continued expansion in the future.
Advancements in Vaccine Technologies
Technological advancements in vaccine development are significantly influencing the Vaccine Contract Development and Manufacturing Organization Market . Innovations, such as mRNA platforms and viral vector technologies, enable rapid prototyping of vaccines against a variety of pathogens. These advancements allow for quicker response times in the face of emerging health crises, enhancing the capabilities of contract development organizations to meet global vaccine needs efficiently.As companies strive to overcome traditional limitations in vaccine development, the integration of novel technologies positions them as pivotal players in the market.
Growing Investment in Biopharmaceutical R
The rise of investment in biopharmaceutical research and development (R) is a major driver for the Vaccine Contract Development and Manufacturing Organization Market . Increased funding from both the public and private sectors seeks to foster innovations in vaccine design and production processes. This influx of capital allows contract manufacturing organizations to expand their facilities, enhance capacities, and optimize operational efficiencies.As a result, they can better cater to the growing demands of vaccine supply chains while maintaining high standards of quality and compliance.
Vaccine Contract Development and Manufacturing Organization Market Segment Insights
Vaccine Contract Development and Manufacturing Organization Market Service Type Insights
The 'Vaccine Contract Development and Manufacturing Organization Market' has witnessed notable developments in its Service Type segment, which comprises critical areas such as Vaccine Development, Vaccine Manufacturing, Regulatory Support, and Quality Control. In 2024, the market valuation for this entire segment will reach approximately 4.63 USD Billion, reflecting ongoing investments and innovations in vaccine solutions. Vaccine Development stands out with a valuation of 1.39 USD Billion, demonstrating its essential role in pioneering new vaccines to cope with emerging health threats.
This segment's importance is underscored by the increasing demand for novel vaccines and the necessity to expedite their development timelines in response to global health challenges.
Vaccine Manufacturing, holding a significant share valued at 2.32 USD Billion, dominates the market due to the growing need for large-scale production capacities to meet global vaccination demands. The shift towards outsourcing manufacturing activities has been a key driver for this segment, as organizations aim to leverage specialized expertise and advanced technologies to enhance production efficiency. On the other hand, Regulatory Support, with a valuation of 0.58 USD Billion, is crucial in ensuring compliance with international guidelines and facilitating faster approvals for new vaccine candidates, thereby playing an integral role in the market’s growth dynamics.
Meanwhile, Quality Control, although smaller at 0.34 USD Billion, represents a vital component in vaccine safety, ensuring that the products meet stringent standards required for public health approval. As the market progresses towards an expected valuation of 12.0 USD Billion by 2035, these segments will continue to evolve, driven by technological advancements, strategic collaborations, and increasing global health awareness, solidifying the 'Vaccine Contract Development and Manufacturing Organization Market revenue' and reflecting the significant trends shaping the industry.
The statistics surrounding these service types illuminate the broad landscape of growth opportunities and highlight the essential aspects of the industry's infrastructure, outlining how these segments interconnect to foster a resilient vaccine ecosystem that responds effectively to global healthcare needs.
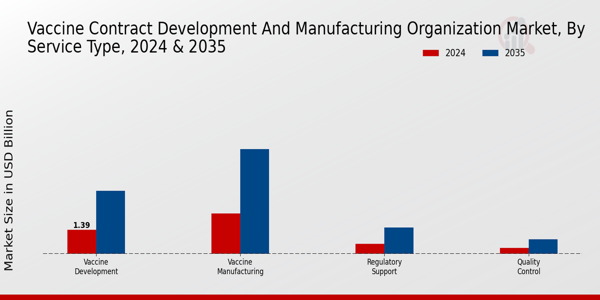
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
Vaccine Contract Development and Manufacturing Organization Market Vaccine Type Insights
The Vaccine Contract Development and Manufacturing Organization Market is projected to reach a valuation of 4.63 USD Billion in 2024, reflecting a robust demand for various Vaccine Types. This market includes crucial categories such as Inactivated Vaccines, Live Attenuated Vaccines, Subunit Vaccines, and DNA Vaccines, each playing a vital role in immunization strategies worldwide. Inactivated Vaccines are often preferred due to their enhanced safety profile, appealing to healthcare providers and patients alike. Live Attenuated Vaccines, on the other hand, provide strong immunity and are recognized for their efficiency in prompt public health responses.
Meanwhile, Subunit Vaccines are becoming increasingly significant due to their specific targeting capabilities and reduced side effects, making them favored choices among manufacturers. DNA Vaccines are witnessing a surge owing to their capacity for rapid development and adaptability, particularly evident in recent health emergencies. Overall, the market growth is fueled by rising vaccination needs, technological advancements, and an increasing emphasis on preventive healthcare across the globe. The Vaccine Contract Development and Manufacturing Organization Market statistics project a strong evolution, highlighting not only the current landscape but also the immense opportunities for innovation and expansion in the years ahead.
Vaccine Contract Development and Manufacturing Organization Market End User Insights
The Vaccine Contract Development and Manufacturing Organization Market is poised for substantial growth, with a projected value of 4.63 USD Billion in 2024. This market is broadly segmented by End Users, which include Pharmaceutical Companies, Biotechnology Companies, Government Organizations, and Research Institutions. Pharmaceutical Companies are significant players in the vaccine development landscape, leveraging contract manufacturing organizations to expedite production and meet demand effectively. Biotechnology Companies also play a crucial role, as they often focus on innovation and bring novel vaccines to the market.
Government Organizations drive initiatives to enhance public health by funding vaccine projects and managing distribution strategies. Research Institutions contribute to the pipeline of vaccine development through critical research and testing, making them a fundamental pillar in the vaccine ecosystem. Collectively, these segments illustrate the dynamic interplay within the Vaccine Contract Development and Manufacturing Organization Market, reflecting its robust structure, growth patterns, and significant contributions to global health outcomes.As the market evolves, understanding these segments will be essential for navigating trends, challenges, and opportunities within the industry.
Vaccine Contract Development and Manufacturing Organization Market Technology Insights
The Vaccine Contract Development and Manufacturing Organization Market is experiencing substantial growth driven by advancements in technology, expected to reach a value of 4.63 USD Billion by 2024. As the market evolves, critical segments such as Classical Vaccination Techniques, Recombinant DNA Technology, mRNA Technology, and Viral Vector Technology emerge as essential components of this landscape.
Classical Vaccination Techniques have a long-standing role in producing vaccines, underpinning their continued popularity. Recombinant DNA Technology is gaining traction due to its ability to create safer and more effective vaccines by manipulating genetic material.mRNA Technology has gained significant importance, particularly following its success in recent vaccine development, showcasing rapid production capabilities and adaptability against emerging pathogens.
Viral Vector Technology is also gaining momentum, renowned for its ability to induce strong immune responses. Together, these segments reflect the diversity and innovation within the Vaccine Contract Development and Manufacturing Organization Market, influencing market trends, growth drivers, challenges, and opportunities in response to global health demands.With evolving needs in vaccine production, these technologies are poised to play a pivotal role in enhancing vaccine effectiveness and production efficiency.
Vaccine Contract Development and Manufacturing Organization Market Regional Insights
The Vaccine Contract Development and Manufacturing Organization Market is projected to exhibit robust growth across various regions, with North America leading the market dynamics. Valued at 2.0 USD Billion in 2024, North America is expected to escalate to 5.2 USD Billion by 2035, showcasing its majority holding in the overall market. Europe follows with a valuation of 1.2 USD Billion in 2024, expected to rise to 3.2 USD Billion, which underscores its significant role in fostering vaccine development and manufacturing capabilities.
The APAC region, valued at 0.8 USD Billion in 2024, is anticipated to grow to 2.2 USD Billion, indicating an emerging market with substantial opportunities driven by increasing healthcare investments.
In contrast, South America and MEA represent the smaller segments, valued at 0.3 USD Billion and 0.33 USD Billion, respectively, in 2024, with projections reaching 0.8 USD Billion by 2035, reflecting a growing interest in vaccine manufacturing amidst regional challenges. The variations in these regional values highlight the distinct market potential and the need for targeted strategies to navigate growth drivers and challenges specific to each area in the Vaccine Contract Development and Manufacturing Organization Market .
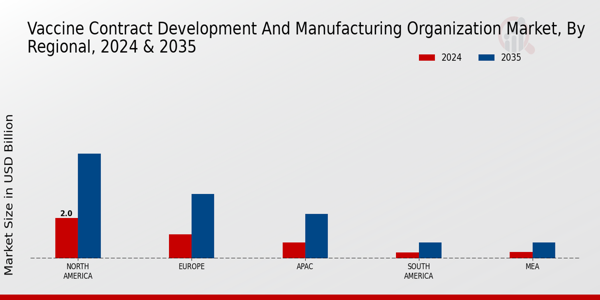
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
Vaccine Contract Development and Manufacturing Organization Market Key Players and Competitive Insights
The Vaccine Contract Development and Manufacturing Organization Market is a crucial component of the biopharmaceutical landscape, fostering collaboration between vaccine developers and specialized organizations to efficiently manage the complexities of vaccine production.
This market thrives on the increasing demand for vaccines driven by global health challenges, such as infectious disease outbreaks and public health initiatives. As the race for innovation in vaccine technology accelerates, companies are seeking strategic partnerships with contract development and manufacturing organizations to leverage their expertise, minimize risk, and reduce time-to-market. The competitive dynamics in this sector are shaped by advancements in bioprocessing technologies, regulatory compliance, and a growing emphasis on flexibility and scalability in manufacturing capabilities.
Boehringer Ingelheim has established a formidable presence in the Vaccine Contract Development and Manufacturing Organization Market, recognized for its robust capabilities in vaccine production and development. The company’s strengths lie in its extensive experience in biologics and vaccine manufacturing, coupled with state-of-the-art facilities that are designed to comply with stringent regulatory standards. Boehringer Ingelheim’s commitment to innovation is reflected in its investment in cutting-edge technologies that enhance production efficiency and product quality.
Furthermore, the organization excels in providing comprehensive services that encompass not only manufacturing but also development and analytical support, enabling clients to optimize their development timelines and regulatory pathways. The company’s global reach and established reputation further enhance its competitive edge, making it a preferred partner for many vaccine developers worldwide.
KBI Biopharma has carved a niche for itself within the Vaccine Contract Development and Manufacturing Organization Market by focusing on providing tailored solutions that address the unique challenges of vaccine development and production. The company's dedication to customer-centric services sets it apart, as it collaborates closely with clients to align on specific project requirements and timelines. KBI Biopharma’s infrastructure is equipped with the latest biomanufacturing technologies, enabling it to produce a wide range of vaccine candidates, from early clinical phases to large-scale commercial manufacturing.
Additionally, their expertise in both mammalian and microbial cell cultures allows KBI Biopharma to cater to diverse biopharmaceutical needs, which adds to its appeal in the vaccine sector. The organization's emphasis on quality assurance, regulatory compliance, and a commitment to delivering reliable results positions it as a strong player in the competitive landscape of vaccine contract development and manufacturing.
Key Companies in the Vaccine Contract Development and Manufacturing Organization Market Include
- Fujifilm Diosynth Biotechnologies
Vaccine Contract Development and Manufacturing Organization Market Developments
Recent developments in the Vaccine Contract Development and Manufacturing Organization Market have seen heightened activity due to an ongoing focus on vaccine production capacities and supply chain enhancements. Companies like Boehringer Ingelheim and WuXi AppTec are increasingly expanding their collaboration efforts, aiming to build robust platforms that facilitate rapid vaccine development and manufacturing. In the merger and acquisition landscape, Samsung Biologics announced plans to acquire a facility from a competitor, while Baxter International is expanding its capabilities through strategic partnerships. KBI Biopharma continues to leverage its expertise to meet rising demand, reflecting an overall growth trajectory across the sector.
Additionally, companies such as Merck and Co. and Catalent are experiencing increased market valuations as they expand their contract manufacturing services amidst a surge in global vaccine deployment efforts. This growing demand is shaped by the competitive landscape and ongoing innovations aimed at enhancing vaccine efficacy, safety, and delivery mechanisms. As regulatory requirements evolve, organizations like Sanofi and Fujifilm Diosynth Biotechnologies are strengthening their RD pipelines to maintain competitive advantage and ensure compliance in a dynamic market environment.
Vaccine Contract Development and Manufacturing Organization Market Segmentation Insights
Vaccine Contract Development and Manufacturing Organization Market Service Type Outlook
Vaccine Contract Development and Manufacturing Organization Market Vaccine Type Outlook
Vaccine Contract Development and Manufacturing Organization Market End User Outlook
Vaccine Contract Development and Manufacturing Organization Market Technology Outlook
- Classical Vaccination Techniques
- Recombinant DNA Technology
Vaccine Contract Development and Manufacturing Organization Market Regional Outlook
| Attribute/Metric Source: |
Details |
| MARKET SIZE 2023 |
4.24(USD Billion) |
| MARKET SIZE 2024 |
4.63(USD Billion) |
| MARKET SIZE 2035 |
12.0(USD Billion) |
| COMPOUND ANNUAL GROWTH RATE (CAGR) |
9.05% (2025 - 2035) |
| REPORT COVERAGE |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| BASE YEAR |
2024 |
| MARKET FORECAST PERIOD |
2025 - 2035 |
| HISTORICAL DATA |
2019 - 2024 |
| MARKET FORECAST UNITS |
USD Billion |
| KEY COMPANIES PROFILED |
Boehringer Ingelheim, KBI Biopharma, Vetter Pharma, PTC Therapeutics, WuXi AppTec, Baxter International, Sanofi, Fujifilm Diosynth Biotechnologies, Samsung Biologics, Merck and Co., Catalent, Aenova, Lonza, Recipharm, Novartis |
| SEGMENTS COVERED |
Service Type, Vaccine Type, End User, Technology, Regional |
| KEY MARKET OPPORTUNITIES |
Increase in vaccine demand, Expansion into emerging markets, Technological advancements in production, Partnerships with biotech firms, Focus on personalized vaccines |
| KEY MARKET DYNAMICS |
Growing vaccine demand, Increasing globalization, Technological advancements, Regulatory challenges, Cost containment pressures |
| COUNTRIES COVERED |
North America, Europe, APAC, South America, MEA |
Frequently Asked Questions (FAQ):
The market is expected to be valued at 4.63 USD Billion in 2024.
By 2035, the market is projected to be valued at 12.0 USD Billion.
The market is expected to grow at a CAGR of 9.05% during the forecast period from 2025 to 2035.
North America is expected to have the largest market value at 2.0 USD Billion in 2024.
The market value for Europe is expected to reach 3.2 USD Billion by 2035.
Major players include Boehringer Ingelheim, WuXi AppTec, Merck and Co., and Sanofi among others.
The market value for Vaccine Manufacturing is expected to be 2.32 USD Billion in 2024.
The market size for Vaccine Development is projected to reach 3.62 USD Billion by 2035.
The Regulatory Support segment is expected to grow to 1.52 USD Billion by 2035.
South America is projected to have a market value of 0.8 USD Billion by 2035.

















