

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Market Size Snapshot
| Year | Value |
|---|---|
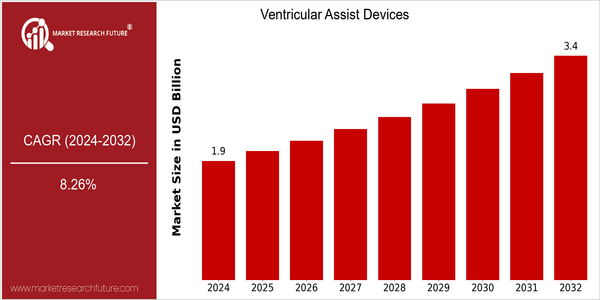
| 2024 | USD 1.95 Billion |
| 2032 | USD 3.39 Billion |
| CAGR (2024-2032) | 8.26 % |
Note – Market size depicts the revenue generated over the financial year
The global Ventricular Assist Devices (VAD) market is poised for significant growth, with a current market size of USD 1.95 billion in 2024, projected to expand to USD 3.39 billion by 2032. This growth trajectory reflects a robust compound annual growth rate (CAGR) of 8.26% over the forecast period. The increasing prevalence of heart failure, coupled with advancements in medical technology, is driving demand for VADs as a critical therapeutic option for patients with severe cardiac conditions. Key factors contributing to this market expansion include the rising geriatric population, which is more susceptible to cardiovascular diseases, and the growing awareness of VADs as a viable alternative to heart transplants. Technological innovations, such as miniaturization of devices and improvements in biocompatible materials, are enhancing the performance and patient outcomes associated with VADs. Major players in the market, including Abbott Laboratories, Medtronic, and Terumo Corporation, are actively engaging in strategic initiatives such as partnerships, investments in R&D, and product launches to strengthen their market position and cater to the evolving needs of healthcare providers and patients.
Regional Market Size
Regional Deep Dive
The Ventricular Assist Devices (VAD) market is experiencing significant growth across various regions, driven by increasing prevalence of heart failure, advancements in technology, and rising awareness about heart health. In North America, the market is characterized by a high adoption rate of innovative devices, robust healthcare infrastructure, and strong reimbursement policies. Europe showcases a diverse landscape with varying regulatory environments and a growing emphasis on minimally invasive procedures. The Asia-Pacific region is witnessing rapid growth due to rising healthcare expenditures and an increasing aging population. Meanwhile, the Middle East and Africa are gradually adopting VAD technology, influenced by improving healthcare systems and government initiatives. Latin America is also emerging as a potential market, driven by increasing investments in healthcare and rising incidences of cardiovascular diseases.
Europe
- The European Union has introduced new regulations aimed at improving the safety and efficacy of medical devices, which is expected to streamline the approval process for VADs and encourage innovation.
- Companies such as Carmat and SynCardia are leading the way in developing next-generation VADs, with a focus on biocompatibility and long-term support for heart failure patients.
Asia Pacific
- Countries like Japan and China are witnessing a surge in VAD adoption due to increasing awareness of heart diseases and government initiatives to improve cardiovascular care.
- Local companies, such as Terumo Corporation, are collaborating with international firms to enhance the availability and affordability of VADs in the region.
Latin America
- Brazil and Mexico are emerging markets for VADs, with increasing investments in healthcare infrastructure and a growing number of specialized cardiac centers.
- Government programs aimed at reducing the burden of cardiovascular diseases are promoting the adoption of VADs, supported by collaborations with global medical device manufacturers.
North America
- The U.S. Food and Drug Administration (FDA) has recently approved several new VAD systems, including the HeartMate 3, which has improved patient outcomes and expanded the market.
- Key players like Abbott and Medtronic are investing heavily in R&D to innovate and enhance the functionality of VADs, focusing on patient-centric designs and remote monitoring capabilities.
Middle East And Africa
- The Gulf Cooperation Council (GCC) countries are investing in advanced healthcare technologies, including VADs, as part of their national health strategies to combat rising cardiovascular diseases.
- Partnerships between local hospitals and international medical device companies are facilitating the introduction of VADs, improving access to life-saving technologies.
Did You Know?
“Approximately 5.7 million adults in the U.S. are living with heart failure, and VADs can significantly improve survival rates and quality of life for these patients.” — American Heart Association
Segmental Market Size
The Ventricular Assist Devices (VAD) segment plays a crucial role in the cardiovascular market, primarily serving patients with severe heart failure. This segment is currently experiencing growth, driven by an increasing prevalence of heart diseases and advancements in device technology. Key factors propelling demand include the rising aging population, which is more susceptible to heart conditions, and the growing acceptance of VADs as a bridge to heart transplants or as destination therapy. Regulatory support, such as expedited approval processes for innovative devices, further enhances market dynamics. Currently, the adoption of VADs is in a mature stage, with leading companies like Abbott and Medtronic spearheading advancements in device design and functionality. Notable regions leading in adoption include North America and Europe, where healthcare infrastructure supports widespread use. Primary applications of VADs include short-term support during surgery and long-term management of chronic heart failure. Trends such as the increasing focus on patient-centric care and technological innovations, including miniaturization and wireless monitoring, are catalyzing growth in this segment, ensuring that VADs remain integral to modern cardiac care.
Future Outlook
The market for Ventricular Assist Devices (VADs) is poised for significant growth from 2024 to 2032, with a projected market value increase from $1.95 billion to $3.39 billion, reflecting a robust compound annual growth rate (CAGR) of 8.26%. This growth trajectory is primarily driven by the rising prevalence of heart failure and the increasing adoption of advanced cardiac therapies. As healthcare systems worldwide continue to prioritize innovative solutions for chronic heart conditions, VADs are expected to see enhanced penetration rates, potentially reaching over 15% of the heart failure patient population by 2032, up from approximately 8% in 2024. This shift will be supported by improved patient outcomes and a growing body of clinical evidence endorsing the efficacy of VADs in both bridge-to-transplant and destination therapy scenarios. Technological advancements will play a crucial role in shaping the future of the VAD market. Innovations such as miniaturization of devices, wireless monitoring capabilities, and biocompatible materials are expected to enhance device performance and patient comfort. Additionally, the integration of artificial intelligence and machine learning in patient management systems will facilitate personalized treatment plans, further driving adoption rates. Policy drivers, including increased reimbursement for VAD procedures and supportive regulatory frameworks, will also contribute to market expansion. As the landscape evolves, stakeholders must remain vigilant to emerging trends, such as the growing emphasis on home healthcare solutions and the potential for VADs to be used in outpatient settings, which will redefine patient care paradigms and expand market opportunities.
Ventricular Assist Devices Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









