-
EXECUTIVE SUMMARY
-
MARKET INTRODUCTION
-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews
- Breakdown of Primary Respondents
-
and Information Gathering Process
-
ForecastingTechniques
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
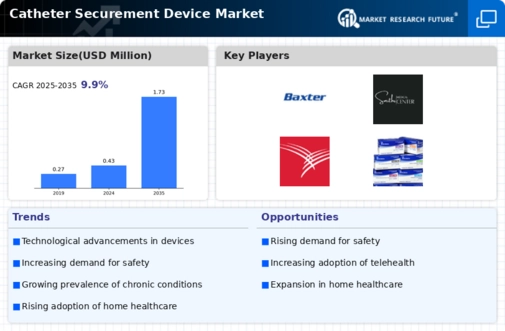
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Porter’s Five Forces Analysis
- Bargaining Power
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
of Suppliers
-
5.1.2.
-
Bargaining Power of Buyers
-
Value Chain Analysis
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Impact on Supply
-
Chain
-
5.3.3.
-
Regional Impact
-
5.3.4.
-
Opportunity and Threat Analysis
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET, BY PRODUCT TYPE
-
Overview
-
Arterial
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
Central Venous
- Peripherally Inserted
-
Central Catheter (PICC)
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Subclavian
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Midlines
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Femoral
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Portal
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Jugular
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Peripheral
- Foley
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Nasogastric Tube
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Endotracheal Tube
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Ventriculoperitoneal
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Continuous Nerve Block
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Chest Drainage Tube
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Abdominal Drainage Tubes
- Percutaneous Endoscopic Gastrostomy
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Jejunal
-
Market Estimates & Forecast, by Country, 2018–2027
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Umbilical
-
Market Estimates & Forecast, by Country, 2018–2027
-
Epidural
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
All-Site Securement
-
Devices
-
Market
-
Estimates & Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
GLOBAL CATHETER SECUREMENT
-
DEVICE MARKET, BY APPLICATION
-
Overview
-
Cardiovascular
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Radiology
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
General Surgery
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Urological
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Respiratory
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
Gastric and Oropharyngeal
-
Market Estimates & Forecast, by Region, 2018–2027
-
Market Estimates &
-
Forecast, by Country, 2018–2027
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET, BY END
-
USER
-
Overview
-
Hospitals
-
& Clinics
-
Market
-
Estimates & Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
Home Healthcare
-
Market Estimates
-
& Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
Diagnostic Centers
-
Market Estimates &
-
Forecast, by Region, 2018–2027
-
Market Estimates & Forecast, by Country, 2018–2027
-
GLOBAL CATHETER SECUREMENT
-
DEVICE MARKET, BY REGION
-
Overview
-
North America
- US
- Rest
-
9.2.2.
-
Canada
-
9.3.
-
Europe
-
9.3.1.
-
Germany
-
9.3.2.
-
France
-
9.3.3.
-
Italy
-
9.3.4.
-
Spain
-
9.3.5.
-
UK
-
of Europe
-
9.4.
-
Asia-Pacific
-
9.4.1.
-
Japan
-
9.4.2.
-
China
-
9.4.3.
-
India
-
9.4.4.
-
Australia
-
9.4.5.
-
South Korea
-
9.4.6.
-
Rest of Asia-Pacific
-
9.5.
-
Rest of the World
-
9.5.1.
-
Middle East
-
9.5.2.
-
Africa
-
9.5.3.
-
Latin America
-
10.
-
COMPANY LANDSCAPE
-
10.1.
-
Overview
-
10.2.
-
Competitive Analysis
-
10.3.
-
Market Share Analysis
-
10.4.
-
Major Growth Strategy in the Global Catheter Securement Device Market
-
Competitive Benchmarking
-
Leading Players
-
in terms of Number of Developments in the Global Catheter Securement Device Market
-
Key
- New ProductLaunch/Service Deployment
- Merger &Acquisition
- Joint Venture
-
developments and Growth Strategies
-
Major Players Financial
- Major Players R&D Expenditure, 2020
-
Matrix
-
10.8.1.
-
Sales & Operating Income, 2020
-
COMPANY PROFILES
-
3M Company
- Company Overview
- Key
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Financials
-
11.1.3.
-
Products Offered
-
11.1.4.
-
Key Developments
-
11.1.5.
-
SWOT Analysis
-
11.1.6.
-
Key Strategies
-
11.2.
-
B. Braun Melsungen AG
-
11.2.1.
-
Company Overview
-
11.2.2.
-
Key Financials
-
11.2.3.
-
Products Offered
-
11.2.4.
-
Key Developments
-
11.2.5.
-
SWOT Analysis
-
11.2.6.
-
Key Strategies
-
11.3.
-
Baxter International, Inc.
-
C.R. Bard, Inc.
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Centurion Medical Products
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Analysis
-
11.5.6.
-
Key Strategies
-
11.6.
-
ConvaTec Group plc
-
11.6.1.
-
Company Overview
-
11.6.2.
-
Key Financials
-
11.6.3.
-
Products Offered
-
11.6.4.
-
Key Developments
-
11.6.5.
-
SWOT Analysis
-
11.6.6.
-
Key Strategies
-
11.7.
-
Merit Medical Systems, Inc.
-
Smiths Medical
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
TIDI Products, LLC
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
M.C. Johnson Company, Inc.
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Analysis
-
11.10.6.
-
Key Strategies
-
11.11.
-
DeRoyal Industries, Inc.
-
Cardinal Health
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
BioDerm Inc.
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Starboard Medical, Inc.
- Company Overview
- Key Financials
- Products Offered
- Key Developments
- SWOT
-
Analysis
-
11.14.6.
-
Key Strategies
-
11.15.
-
Pepper Medical
-
11.15.1.
-
Company Overview
-
11.15.2.
-
Key Financials
-
11.15.3.
-
Products Offered
-
11.15.4.
-
Key Developments
-
11.15.5.
-
SWOT Analysis
-
11.15.6.
-
Key Strategies
-
12.
-
APPENDIX
-
12.1.
-
References
-
12.2.
-
Related Reports
-
LIST
-
OF TABLES
-
TABLE
-
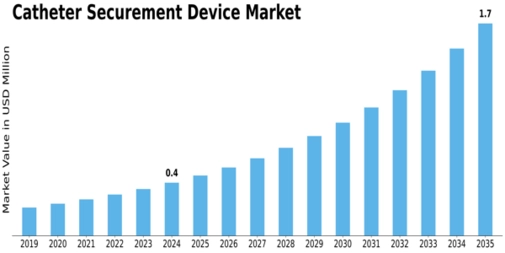
GLOBAL CATHETER SECUREMENT DEVICE MARKET SYNOPSIS, 2018–2027
-
GLOBAL CATHETER SECUREMENT
-
DEVICE MARKET ESTIMATES & FORECAST, 2018–2027 (USD MILLION)
-
GLOBAL CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
GLOBAL CATHETER
-
SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
GLOBAL CATHETER
-
SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
NORTH AMERICA: CATHETER
-
SECUREMENT DEVICE MARKET, BY COUNTRY, 2018–2027 (USD MILLION)
-
NORTH AMERICA:CATHETER
-
SECUREMENT DEVICE MARKET, BY PRODUCT TYPE, 2018–2027 (USD MILLION)
-
NORTH AMERICA:CATHETER
-
SECUREMENT DEVICE MARKET, BY APPLICATION, 2018–2027 (USD MILLION)
-
NORTH AMERICA:
-
CATHETER SECUREMENT DEVICE MARKET, BY END USER, 2018–2027 (USD MILLION)
-
US:CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
US:CATHETER
-
SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
US: CATHETER
-
SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
CANADA:CATHETER SECUREMENT
-
DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
CANADA:CATHETER SECUREMENT DEVICE MARKET,BY
-
APPLICATION, 2018–2027 (USD MILLION)
-
CANADA: CATHETER SECUREMENT DEVICE MARKET,BY
-
END USER, 2018–2027 (USD MILLION)
-
EUROPE: CATHETER SECUREMENT DEVICE MARKET,BY COUNTRY,
-
EUROPE: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027
-
(USD MILLION)
-
TABLE
-
EUROPE: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD
-
MILLION)
-
TABLE
-
EUROPE: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
GERMANY: CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
GERMANY: CATHETER
-
SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
GERMANY: CATHETER
-
SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
FRANCE: CATHETER SECUREMENT
-
DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
FRANCE: CATHETER SECUREMENT DEVICE MARKET,BY
-
APPLICATION, 2018–2027 (USD MILLION)
-
FRANCE: CATHETER SECUREMENT DEVICE MARKET,BY
-
END USER, 2018–2027 (USD MILLION)
-
ITALY: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT
-
TYPE,2018–2027 (USD MILLION)
-
ITALY: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION,
-
ITALY: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027
-
(USD MILLION)
-
TABLE
-
SPAIN: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD
-
MILLION)
-
TABLE
-
SPAIN: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD
-
MILLION)
-
TABLE
-
SPAIN: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
UK: CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
UK: CATHETER
-
SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
UK: CATHETER
-
SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
REST OF EUROPE: CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
REST OF EUROPE:
-
CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
REST OF EUROPE:
-
CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
ASIA-PACIFIC:
-
CATHETER SECUREMENT DEVICE MARKET,BY COUNTRY, 2018–2027 (USD MILLION)
-
ASIA-PACIFIC:
-
CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
ASIA-PACIFIC:
-
CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
ASIA-PACIFIC:
-
CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
JAPAN: CATHETER
-
SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
JAPAN: CATHETER
-
SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
JAPAN: CATHETER
-
SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
CHINA: CATHETER SECUREMENT
-
DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
CHINA: CATHETER SECUREMENT DEVICE MARKET,BY
-
APPLICATION, 2018–2027 (USD MILLION)
-
CHINA: CATHETER SECUREMENT DEVICE MARKET,BY
-
END USER, 2018–2027 (USD MILLION)
-
INDIA: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT
-
TYPE,2018–2027 (USD MILLION)
-
INDIA: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION,
-
INDIA: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027
-
(USD MILLION)
-
TABLE
-
AUSTRALIA: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027
-
(USD MILLION)
-
TABLE
-
AUSTRALIA: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027
-
(USD MILLION)
-
TABLE
-
AUSTRALIA: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD
-
MILLION)
-
TABLE
-
SOUTH KOREA: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027
-
(USD MILLION)
-
TABLE
-
SOUTH KOREA: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027
-
(USD MILLION)
-
TABLE
-
SOUTH KOREA: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD
-
MILLION)
-
TABLE
-
REST OF ASIA-PACIFIC: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF ASIA-PACIFIC: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF ASIA-PACIFIC: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF THE WORLD: CATHETER SECUREMENT DEVICE MARKET,BY REGION, 2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF THE WORLD: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF THE WORLD: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027
-
(USD MILLION)
-
TABLE
-
REST OF THE WORLD: CATHETER SECUREMENT DEVICE MARKET, BY END USER, 2018–2027
-
(USD MILLION)
-
TABLE
-
MIDDLE EAST: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE, 2018–2027
-
(USD MILLION)
-
TABLE
-
MIDDLE EAST: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027
-
(USD MILLION)
-
TABLE
-
MIDDLE EAST: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD
-
MILLION)
-
TABLE
-
AFRICA: CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD
-
MILLION)
-
TABLE
-
AFRICA: CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD
-
MILLION)
-
TABLE
-
AFRICA: CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
LATIN AMERICA:
-
CATHETER SECUREMENT DEVICE MARKET,BY PRODUCT TYPE,2018–2027 (USD MILLION)
-
LATIN AMERICA:
-
CATHETER SECUREMENT DEVICE MARKET,BY APPLICATION, 2018–2027 (USD MILLION)
-
LATIN AMERICA:
-
CATHETER SECUREMENT DEVICE MARKET,BY END USER, 2018–2027 (USD MILLION)
-
LIST OF FIGURES
-
RESEARCH PROCESS
-
MARKET STRUCTURE
-
FOR THE GLOBAL CATHETER SECUREMENT DEVICE MARKET
-
MARKET DYNAMICS FOR THE GLOBAL CATHETER
-
SECUREMENT DEVICE MARKET
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET SHARE, BY PRODUCT
-
TYPE,2020(%)
-
FIGURE
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET SHARE, BY APPLICATION, 2020 (%)
-
GLOBAL CATHETER
-
SECUREMENT DEVICE MARKET SHARE, BY END USER, 2020(%)
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET
-
SHARE, BY REGION, 2020(%)
-
NORTH AMERICA: CATHETER SECUREMENT DEVICE MARKET SHARE, BY
-
REGION, 2020(%)
-
FIGURE
-
EUROPE: CATHETER SECUREMENT DEVICE MARKET SHARE, BY REGION, 2020(%)
-
ASIA-PACIFIC:
-
CATHETER SECUREMENT DEVICE MARKET SHARE, BY REGION, 2020(%)
-
REST OF THE WORLD: CATHETER SECUREMENT
-
DEVICE MARKET SHARE, BY REGION, 2020(%)
-
GLOBAL CATHETER SECUREMENT DEVICE MARKET: COMPANY
-
SHARE ANALYSIS, 2020(%)
-
3M COMPANY: FINANCIAL OVERVIEW
-
3M COMPANY: SWOT ANALYSIS
-
B. BRAUN MELSUNGEN
-
AG:FINANCIAL OVERVIEW
-
FIGURE
-
B. BRAUN MELSUNGEN AG:SWOT ANALYSIS
-
BAXTER INTERNATIONAL, INC.:FINANCIAL OVERVIEW
-
BAXTER INTERNATIONAL,
-
INC.:SWOT ANALYSIS
-
FIGURE
-
C.R. BARD, INC.:FINANCIAL OVERVIEW
-
C.R. BARD, INC.:SWOT ANALYSIS
-
CENTURION MEDICAL PRODUCTS:FINANCIAL
-
OVERVIEW
-
FIGURE
-
CENTURION MEDICAL PRODUCTS:SWOT ANALYSIS
-
CONVATEC GROUP PLC:FINANCIAL OVERVIEW
-
CONVATEC GROUP
-
PLC:SWOT ANALYSIS
-
FIGURE
-
MERIT MEDICAL SYSTEMS, INC.:FINANCIAL OVERVIEW
-
MERIT MEDICAL SYSTEMS, INC.:SWOT ANALYSIS
-
SMITHS MEDICAL:
-
FINANCIAL OVERVIEW
-
FIGURE
-
SMITHS MEDICAL: SWOT ANALYSIS
-
TIDI PRODUCTS, LLC: FINANCIAL OVERVIEW
-
TIDI PRODUCTS, LLC:
-
SWOT ANALYSIS
-
FIGURE
-
M.C. JOHNSON COMPANY, INC.:FINANCIAL OVERVIEW
-
M.C. JOHNSON COMPANY, INC.:SWOT ANALYSIS
-
DEROYAL INDUSTRIES,
-
INC.:FINANCIAL OVERVIEW
-
DEROYAL INDUSTRIES, INC.:SWOT ANALYSIS
-
CARDINAL HEALTH:FINANCIAL
-
OVERVIEW
-
FIGURE
-
CARDINAL HEALTH:SWOT ANALYSIS
-
BIODERM INC.:FINANCIAL OVERVIEW
-
BIODERM INC.:SWOT ANALYSIS
-
STARBOARD MEDICAL,
-
INC.:FINANCIAL OVERVIEW
-
STARBOARD MEDICAL, INC.:SWOT ANALYSIS
-
PEPPER MEDICAL:FINANCIAL OVERVIEW
-
PEPPER MEDICAL:SWOT
-
ANALYSIS






Leave a Comment