Market Trends
Key Emerging Trends in the Arterial Blood Collection Devices Market
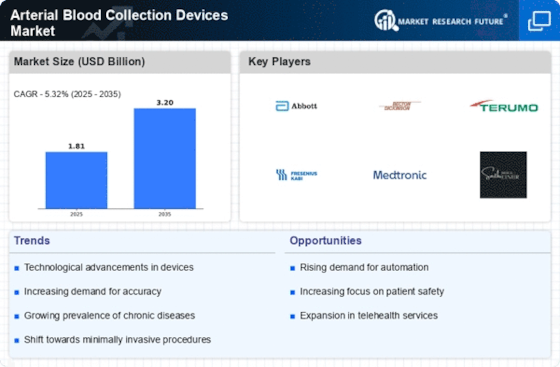
The arterial blood collection devices market is experiencing notable trends influenced by factors such as technological advancements, increasing prevalence of chronic diseases, and a growing focus on patient safety and comfort. One significant trend in this market is the development of innovative arterial blood collection devices that offer improved ease of use, accuracy, and patient comfort. Manufacturers are introducing new designs and materials for arterial blood collection needles, syringes, and catheters to minimize pain, trauma, and complications associated with arterial punctures. These advancements enhance the overall experience for both patients and healthcare providers, driving the adoption of arterial blood collection devices in clinical settings.
Moreover, there is a growing demand for arterial blood gas (ABG) testing and point-of-care (POC) diagnostic solutions, driving the expansion of the arterial blood collection devices market. ABG analysis plays a critical role in the assessment and management of patients with respiratory and metabolic disorders, sepsis, and critical illnesses. As healthcare providers strive to improve patient outcomes and optimize treatment decisions, there is increasing reliance on POC testing devices that enable rapid, accurate, and real-time measurement of arterial blood gases, electrolytes, and metabolic parameters. This trend is driving the development of arterial blood collection devices compatible with POC analyzers and automated systems, facilitating seamless integration into clinical workflows and enhancing efficiency in critical care settings.
Furthermore, the arterial blood collection devices market is witnessing increasing adoption in non-traditional healthcare settings such as ambulatory care centers, home healthcare, and emergency medical services. As healthcare delivery models evolve and decentralize, there is growing demand for portable, user-friendly arterial blood collection devices that can be used outside of traditional hospital settings. This includes handheld arterial blood gas analyzers, pre-packaged arterial blood collection kits, and disposable arterial catheters designed for ease of use and safety in non-clinical environments. These devices enable timely and accurate assessment of patients' respiratory and metabolic status, facilitating rapid decision-making and appropriate interventions in diverse healthcare settings.
Additionally, regulatory changes and quality standards are shaping the arterial blood collection devices market, particularly in terms of safety, sterility, and traceability requirements. Regulatory agencies such as the FDA and European Medicines Agency (EMA) are implementing stricter regulations and guidelines for the design, manufacturing, and labeling of arterial blood collection devices to ensure patient safety and product quality. This includes requirements for biocompatibility testing, sterilization validation, and risk management processes to mitigate potential hazards associated with arterial blood collection procedures. Furthermore, healthcare organizations are implementing quality management systems and accreditation standards such as ISO 15189 and Clinical Laboratory Improvement Amendments (CLIA) to ensure compliance with best practices and quality standards for arterial blood gas testing and diagnostic services.
Moreover, there is a growing emphasis on sustainability and environmental stewardship in the arterial blood collection devices market, driven by increasing awareness of healthcare-related waste and carbon footprint. Manufacturers are exploring eco-friendly materials, packaging solutions, and disposal methods to minimize the environmental impact of arterial blood collection devices throughout their lifecycle. This includes the use of recyclable materials, bio-based polymers, and reduced packaging waste in product design and manufacturing processes. Additionally, there is growing interest in reusable arterial blood collection devices and accessories that can be sterilized and reprocessed for multiple uses, reducing the volume of single-use medical waste generated in healthcare facilities.


















Leave a Comment