Market Analysis
In-depth Analysis of Asia Pacific Respirtory Therapeutic Devices Market Industry Landscape
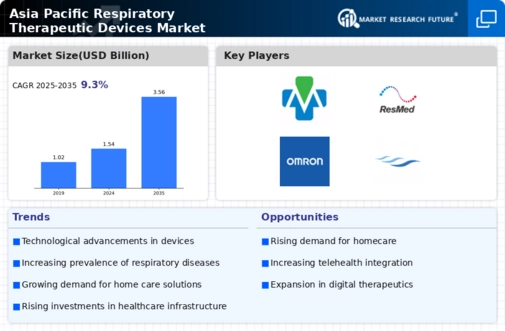
The Asia-Pacific respiratory therapeutic devices market is a dynamic landscape driven by several key factors shaping its growth and evolution. The market reports huge traction due to the escalating occurrence of respiratory problems across the Asia-Pacific place. Factors such as pollutants, lifestyle adjustments, and an aging population contribute to the growing incidence of conditions like chronic obstructive pulmonary disease (COPD) and allergies. Ongoing advancements in respiratory therapeutic devices play a pivotal function in marketplace dynamics. Innovations in inhalation gadgets, nebulizers, and ventilators, incorporating capabilities that include portability, person-friendly interfaces, and progressed efficacy, force adoption, and contribute to the overall marketplace boom. The getting old demographic in Asia-Pacific is a substantial element influencing the demand for respiratory therapeutic devices. With an increasingly aged populace, there's a parallel rise in the superiority of respiratory illnesses, necessitating the use of advanced healing answers for effective control. Worsening air quality throughout several Asia-Pacific countries is an essential driving force for the respiration gadgets marketplace. High levels of air pollution contribute to respiratory ailments, developing a pressing need for healing interventions and breathing aid devices. A growing trend toward domestic healthcare answers in the Asia-Pacific vicinity similarly propels the demand for transportable and easy-to-use respiratory gadgets. Patients select the benefit and comfort of handling breathing conditions at home, fostering the marketplace increase for domestic-based healing gadgets. Strategic collaborations and partnerships between key gamers also influence market dynamics. Collaborative efforts in studies and development, in addition to distribution agreements, contribute to a much better marketplace presence and the creation of revolutionary respiratory healing answers. The regulatory landscape considerably shapes the market dynamics. Compliance with regional health rules, adherence to first-rate standards, and acquiring vital approvals for respiratory therapeutic devices are critical elements impacting marketplace access and sustained increase. Economic factors, including healthcare expenditure and affordability, play a function in marketplace dynamics. The economic prosperity of nations inside the Asia-Pacific location affects the purchasing power of individuals and healthcare institutions, affecting the adoption fee of respiratory therapeutic devices. Intense competition among market players fosters innovation and charge competitiveness. Key groups continually strive to benefit a competitive side via the introduction of novel merchandise, green distribution channels, and strategic advertising projects, thereby contributing to the general dynamism of the Asia-Pacific respiratory therapeutic devices market.















Leave a Comment