

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Biologics Market Size Snapshot
| Year | Value |
|---|---|
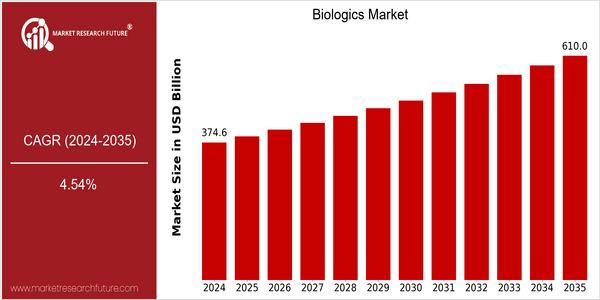
| 2024 | USD 374.57 Billion |
| 2035 | USD 610.0 Billion |
| CAGR (2025-2035) | 4.54 % |
Note – Market size depicts the revenue generated over the financial year
The recombinant DNA market is expected to grow from $374.5 billion in 2024 to $600.0 billion by 2035. A compound annual growth rate (CAGR) of 4.54% from 2025 to 2035 indicates a robust growth in this industry. The growing number of chronic diseases and the growing demand for individualized medicine are the main driving forces of this market. The development of biopharmaceutical technology, such as monoclonal antibodies and gene therapy, also plays an important role in the development of this industry, and offers a variety of new treatment options for patients, thus driving the market. The three major companies in the recombinant DNA industry, namely Amgen, Genentech and AbbVie, have made significant investments in research and development and are expanding their product portfolios. Also, strategic cooperation such as alliances and joint ventures are very popular, such as the recent alliance between Pfizer and BioNTech to develop mRNA-based drugs. The efforts of these companies not only strengthen the competition in the industry, but also promote the development of the industry by promoting the entry of new biological products.
Regional Deep Dive
The market for biopharmaceuticals is experiencing strong growth in all regions, driven by the advancements in biotechnology, the increasing prevalence of chronic diseases and the increasing demand for individualized medicine. In North America, the market is characterized by a strong regulatory framework, high R & D spending and a strong presence of leading biopharmaceutical companies. Europe is a varied landscape with a focus on innovation and a supportive regulatory framework, while the Asia-Pacific region is rapidly emerging due to increasing healthcare expenditure and a growing patient population. The Middle East and Africa face unique challenges, such as the variability of regulatory standards and the disparity between economic development, but are gradually adopting biopharmaceuticals to improve the quality of life. Latin America is seeing a strong increase in the use of biopharmaceuticals, which is driven by government initiatives and public-private partnerships to improve access to and affordability of medicines.
North America
- The Food and Drug Administration has recently accelerated its approval process for biosimilars. The hope is that this will increase competition, reduce costs for patients, and widen access to these treatments.
- Genentech and Amgen are investing heavily in gene therapy and monoclonal antibodies, resulting in new treatment options for diseases such as cancer and autoimmune disorders.
- A number of highly developed research institutions and a fertile venture capital environment in the United States are stimulating innovation in biological products, and collaboration between academics and industry is leading to the development of new products.
Europe
- The European Medicines Agency (EMEA) has adopted new guidelines for the development of advanced therapy medicinal products (ATMPs), which are expected to simplify the approval process for such innovative biological medicines.
- The United Kingdom and Germany are leading the way in implementing this new way of treating patients, integrating the genetic information into everyday clinical practice and thereby improving the efficacy of biologicals.
- The European Union’s Horizon 2020 programme is sponsoring a number of research projects in the field of biotechnology, encouraging cooperation between the member states and fostering innovation.
Asia-Pacific
- China is rapidly becoming a major hub for the manufacture of biologicals. The Chinese government is actively promoting biotechnology and has invested heavily in local biopharmaceutical companies.
- Biosimilars, the new field of drug discovery, is growing fast in India, thanks to the regulatory changes and the government’s emphasis on affordable health care. And Biocon is one of the leading players.
- In Japan and Australia, where the number of chronic diseases is increasing, demand for biologicals is pushing the health systems to adapt and to include these therapies in the standard treatment protocol.
MEA
- It is the task of the African Union to ensure that the exploitation of the flora and fauna of the African continent is not neglected. The African Union’s Agenda 2063 places a high priority on the importance of biotechnology in the health sector. As a result, there are now substantial research and development investments in biotechnology on
- The South African regulatory framework is being designed to facilitate the registration of biosimilars. This will be expected to enhance market access and patient care.
- The region is faced with many problems such as the lack of health facilities and the varying availability of drugs. Various initiatives by the World Health Organization are trying to improve the provision of health care and the access to new drugs.
Latin America
- Brazil's National Health Surveillance Agency (ANVISA) has shortened the approval process for biosimilars, which is expected to increase the availability of generic drugs in the market.
- Local governments and international pharmaceutical companies are collaborating to develop these drugs, and thereby improving access to health care for underserved populations.
- Latin America, with its high rate of chronic diseases, has a strong demand for biological products, which the health systems are adapting to and integrating into their standard therapeutic protocol.
Did You Know?
“Biotech drugs have constituted nearly one-third of all new drugs licensed by the Food and Drug Administration in recent years. This reflects their growing importance in modern medicine.” — FDA Annual Drug Approvals Report
Segmental Market Size
The biopharmaceuticals market is a dynamic submarket of the pharmaceutical industry. It is currently undergoing a strong upward trend, owing to the increasing demand for new therapies. The factors driving this growth are the growing number of chronic diseases, advances in biotechnology, and the growing importance of individualized medicine. These factors are reinforced by regulatory policies, such as the accelerated approval of biological products, which facilitate the faster availability of life-saving treatments.
At the present time, the use of biotechnology is at its zenith. Monoclonal antibodies are the mainstay of the industry, and the development of biosimilars is a major focus of the efforts of the major companies. The most important applications are in oncology, autoimmune diseases, and rare diseases, where monoclonal antibodies offer targeted therapies. The pandemic flu has highlighted the importance of biologicals in the development of vaccines and the treatment of influenza. CRISPR and the development of advanced biomanufacturing methods are reshaping this industry, offering more efficient and innovative products.
Future Outlook
From 2024 to 2035, the biosimilars market is expected to reach $601 billion, with a CAGR of 4.54%. The growth of this market is due to the increasing occurrence of chronic diseases, the development of biotechnology and the growing interest in individual medicine. The world's health systems will continue to favour the development of new therapies, and biosimilars will take a larger share of the pharmaceutical market, with a penetration rate of around 30% in 2035 compared to about 20% in 2024. This is due to the growing demand for monoclonal antibodies, gene therapies and biosimilars, which are becoming an integral part of treatment in a large number of therapeutic areas.
In addition, the development of the next generation of genetic sequencing and the CRISPR gene-editing technique are expected to improve the efficacy and safety of these products, which will drive market growth. Regulatory support and increased R & D investment will also help to promote the development of new biological products. Also, the integration of artificial intelligence into drug discovery and the increasing focus on the development of sustainable biomanufacturing processes will change the market landscape. The industry will evolve, and the stakeholders need to be agile to seize opportunities and overcome the high cost of producing and the complex regulatory environment.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 7.6% (2023-2030) |
Biologics Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









