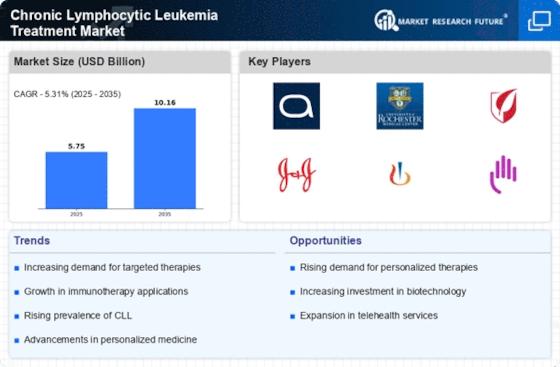
Top Industry Leaders in the Chronic Lymphocytic Leukemia Treatment Market

Pirtobrutinib (Rozlytrek®): In December 2023, the FDA granted accelerated approval to pirtobrutinib for adults with CLL or small lymphocytic lymphoma (SLL) who previously received at least two lines of prior therapy, including a BTK inhibitor and a BCL2 inhibitor. This signifies the first non-covalent BTK inhibitor approved for CLL and represents a significant milestone for targeted therapy options.
Venetoclax (Venclexta®): In October 2023, the FDA approved venetoclax in combination with obinutuzumab for the frontline treatment of patients with CLL and a specific genetic mutation (17p deletion). This expands the use of venetoclax beyond relapsed/refractory CLL and offers a highly effective option for newly diagnosed patients.
Zanubrutinib (Brukinsa®): Positive results from the Phase 3 ELEVATE-RR trial evaluating zanubrutinib in combination with venetoclax for previously untreated CLL were presented at ASCO 2023. This combination demonstrated superior progression-free survival and minimal residual disease compared to standard chemoimmunotherapy.
List of Chronic Lymphocytic Leukemia Treatment Key Companies in the Market
- Hoffmann-La Roche Ltd
- GlaxoSmithKline Plc
- Genmab A/S
- Teva Pharmaceutical Industries Ltd
- Genentech Inc
- Genzyme Corporation
- AbbVie Inc
- Gilead
- Novartis AG
- Johnson & Johnson Services Inc
- AstraZeneca
- TG Therapeutics Inc
- Ziopharm Oncology Inc