

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
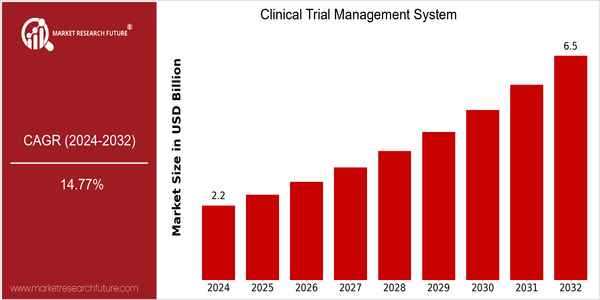
Market Size Snapshot
| Year | Value |
|---|---|
| 2024 | USD 2.16 Billion |
| 2032 | USD 6.5 Billion |
| CAGR (2024-2032) | 14.77 % |
Note – Market size depicts the revenue generated over the financial year
In the clinical trial management system (CTMS) market, the current market size is estimated to be $ 2,165 million in 2024, and is expected to reach $ 6,565 million in 2032. The average annual growth rate during this period is 14.77 percent. The main growth drivers are the increasing complexity of clinical trials and the increasing demand for efficient management solutions. In order to achieve higher efficiency in clinical trials, pharmaceutical and biotech companies need to adopt advanced clinical trial management systems. Several factors have driven the growth of the market, such as the integration of artificial intelligence and machine learning technology to enhance the data management and analysis capabilities. The increased emphasis on patient-centric clinical trial design and regulatory compliance also encourages companies to adopt advanced clinical trial management systems. Medidata, Veeva, and Oracle, as the main players in the market, are actively involved in strategic cooperation and product innovation, in order to enhance their market share. Recent strategic cooperation is expected to increase the scalability and accessibility of clinical trial management systems, which will further drive the market growth.
Regional Market Size
Regional Deep Dive
The clinical trial management system (CTMS) market is experiencing significant growth in different regions, driven by the growing complexity of clinical trials, the need for regulatory compliance, and the demand for efficient data management solutions. Each region has its own set of unique characteristics, which are influenced by local regulations, technological advancements, and the state of the healthcare system. North America is a leading innovator in clinical trial management systems (CTMS), while Europe is focused on regulatory compliance. Asia-Pacific is a fast-growing region, driven by a large patient population and increased investment in medical technology. The Middle East and Africa is growing because of government initiatives to improve the state of the healthcare system, while Latin America is gaining from strategic alliances to enhance its clinical trial capabilities.
Europe
- The European Medicines Agency (EMA) is actively working on the Clinical Trials Regulation (CTR), which aims to simplify the approval process for clinical trials across EU member states, thereby increasing the demand for integrated CTMS solutions.
- Companies like Oracle and Bioclinica are expanding their presence in Europe, focusing on compliance with local regulations and offering tailored solutions to meet the diverse needs of European clinical trial sponsors.
Asia Pacific
- Countries like China and India are witnessing a surge in clinical trials due to favorable regulatory environments and government incentives, leading to increased investments in CTMS technologies.
- Local firms such as Wuxi AppTec and Syneos Health are collaborating with global pharmaceutical companies to enhance clinical trial efficiency, leveraging CTMS to manage large-scale studies effectively.
Latin America
- Brazil's National Health Surveillance Agency (ANVISA) has implemented new regulations to facilitate clinical trials, prompting local pharmaceutical companies to invest in CTMS for better compliance and efficiency.
- Partnerships between local CROs and global pharmaceutical companies are on the rise, with firms like Clinipace and PPD focusing on enhancing clinical trial capabilities through advanced CTMS technologies.
North America
- The U.S. Food and Drug Administration (FDA) has introduced new guidelines to streamline the clinical trial process, encouraging the adoption of digital solutions like CTMS to enhance data integrity and patient safety.
- Key players such as Medidata Solutions and Veeva Systems are investing heavily in cloud-based CTMS platforms, which are becoming increasingly popular among pharmaceutical companies for their scalability and ease of use.
Middle East And Africa
- The UAE government has launched initiatives to position the region as a hub for clinical research, which is driving the adoption of CTMS among local healthcare providers and research institutions.
- Organizations like the African Society for Clinical Pharmacy are advocating for better clinical trial management practices, which is increasing awareness and demand for CTMS solutions across the continent.
Did You Know?
“Approximately 80% of clinical trials are conducted outside the United States, highlighting the growing importance of global CTMS solutions to manage diverse regulatory environments and patient populations.” — Clinical Trials Transformation Initiative (CTTI)
Segmental Market Size
This market is called the clinical trial management system (CTMS) and it is growing rapidly. There are several reasons for this: the increasing complexity of clinical trials, which requires more and more robust management solutions, the rising importance of regulatory compliance with the increasingly stringent guidelines of the FDA and EMA. Also, the trend towards decentralization of clinical trials is pushing companies to adopt advanced clinical trial management systems that facilitate remote monitoring and data collection. The clinical trial management system market is now moving from pilot to scale. The market leaders are Medidata and Veeva. These companies focus on managing clinical trial protocols, patient recruitment and data management, especially in the areas of oncology and rare diseases. The pandemic of COVD-19 has accelerated the move towards digital solutions. The evolution of artificial intelligence and machine learning is shaping the clinical trial management system, improving data analysis and operational efficiency.
Future Outlook
The clinical trial management system market is expected to grow from $2.1 billion to $6.5 billion by 2032, a CAGR of 14.77%. The complexity of clinical trials, the need for efficient trial management, and the need for regulatory compliance will drive this growth. As the pharma and biotech industry continues to invest in R&D, the penetration of clinical trial management systems is expected to increase. By 2032, the penetration rate of CTMS in medium-sized and large organizations is expected to reach over 70%, compared to an estimated 40% in 2024. Artificial intelligence and machine learning integration in clinical trial management systems will enhance data analysis, optimize trial operations, and improve patient recruitment. In addition, the increasing emphasis on patient-centric trial design and decentralized clinical trials will increase the demand for advanced clinical trial management systems. Also, the stricter regulatory environment and the increasing transparency of clinical research will also drive the demand for advanced clinical trial management systems. These factors will converge to form a clinical trial management system market that not only meets the needs of the industry but also anticipates future challenges in clinical trial management.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Market Size Value In 2022 | USD 1.36 billion |
| Growth Rate | 13.40% (2022-2030) |
Clinical Trial Management System Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









