Market Trends
Key Emerging Trends in the Corneal Cross Linking Devices Market
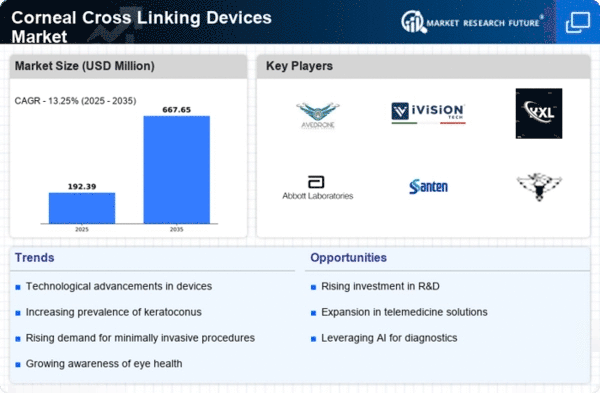
The global market for Corneal Cross-linking (CXL) devices is poised for growth, primarily fueled by the rising incidence and prevalence of keratoconus disorders on a global scale and advancements in healthcare infrastructure. However, this growth may face hindrances due to challenges such as a lack of awareness and potential side effects associated with the treatment. Conversely, opportunities for substantial growth exist in the market through ongoing product development and launches.
The escalating prevalence of keratoconus disorders worldwide stands out as a significant driver for the growth of the Corneal Cross-linking (CXL) devices market. A meta-analysis study conducted by Hashemi et al, funded by the Noor Research Center for Ophthalmic Epidemiology, revealed that the prevalence of keratoconus in the entire population was 1.38 per 1000 population. This suggests a noteworthy occurrence of keratoconus, and recent years have witnessed a surge in reported cases of this disorder. Notably, variations in prevalence are observed among populations due to environmental factors and ethnic differences.
Geographical locations characterized by abundant sunshine and hot weather, such as India and the Middle East, are expected to exhibit higher prevalence rates compared to regions with cooler climates and less sunshine, like Finland, Denmark, Japan, and other colder countries. This geographical distinction is linked to the environmental conditions that may contribute to the development of keratoconus. Additionally, numerous studies indicate a higher prevalence among Asian subjects compared to white Caucasians. As these trends persist, the increasing prevalence of keratoconus disorders is projected to be a sustained driver for the growth of the Corneal Cross-linking (CXL) devices market.
However, despite the positive momentum, challenges exist that may impede the global Corneal Cross-linking (CXL) devices market. One such challenge is the lack of awareness about keratoconus disorders and the treatment options available. Addressing this issue requires concerted efforts in education and awareness campaigns to ensure timely diagnosis and treatment. Moreover, potential side effects associated with corneal crosslinking procedures are a concern that needs to be effectively communicated to both healthcare providers and patients.
On a positive note, the global market presents growth opportunities through continuous product development and launches. As technology and medical science progress, the introduction of innovative devices and procedures enhances the effectiveness of corneal crosslinking, making it a more appealing option for healthcare professionals and patients alike. This innovation-driven approach is expected to create a conducive environment for the Corneal Cross-linking (CXL) devices market, allowing for sustained growth and the provision of advanced treatment options.


















Leave a Comment