

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Market Size Snapshot
| Year | Value |
|---|---|
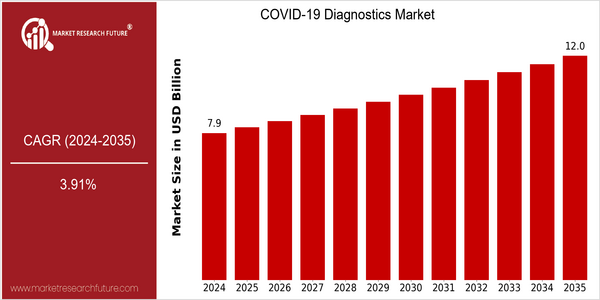
| 2024 | USD 7.87 Billion |
| 2035 | USD 12.0 Billion |
| CAGR (2025-2035) | 3.91 % |
Note – Market size depicts the revenue generated over the financial year
COVID-19 is a new diagnostic method which has been developed to distinguish between the ailment and other similar disorders. It is a rapidly growing industry and the market is estimated to reach a value of $11.8 billion in 2035. The CAGR for the period 2025 to 2035 is 3.91%. The market growth is mainly due to the increasing need for accurate diagnostics, the development of diagnostics and the increasing prevalence of infectious diseases. As health care systems continue to focus on rapid and accurate diagnostics, the demand for new diagnostic methods will increase significantly. The main technological trends driving this market are the development of point-of-care devices, the integration of artificial intelligence into diagnostics and the improvement of molecular diagnostics. These are the areas where the major companies are focusing their efforts, and companies such as Abbott Laboratories, Roche Diagnostics and Thermo Fisher Scientific are concentrating their efforts on strategic initiatives, such as entering into new collaborations, increasing their investment in R & D and launching new products to meet the changing needs of health care systems. These efforts not only strengthen their position in the market, but also help to increase the overall growth of the COVID-19 diagnostics market.
Regional Market Size
Regional Deep Dive
The COVID-19 diagnostics market has shown considerable fluctuations in different regions of the world, influenced by the ongoing pandemic and the need for rapid diagnostics. The North American market is characterized by the most developed health care system, with a high level of research and development, and by the presence of the largest market players. The European market has a variety of regulatory environments, with different degrees of state support and technological innovation. The Asia-Pacific market is growing rapidly, with rising health care expenditures and increasing demand for point-of-care testing. Middle East and Africa have limited access to health care, while Latin America is focusing on improving diagnostics to help control outbreaks.
Europe
- The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which aims to enhance the safety and performance of diagnostic tests, pushing companies like Roche and Siemens Healthineers to innovate and comply with stricter standards.
- Countries like Germany and the UK have invested heavily in rapid testing initiatives, with the UK government launching the 'Test and Trace' program, which has significantly increased the availability of COVID-19 tests across the region.
Asia Pacific
- China has emerged as a leader in COVID-19 diagnostics, with companies like BGI Genomics and Sino Biological developing and distributing large volumes of testing kits, contributing to the region's rapid response to the pandemic.
- India's government has launched initiatives to boost local manufacturing of diagnostic kits, with the 'Make in India' program encouraging companies like Mylab Discovery Solutions to produce affordable testing solutions for the domestic market.
Latin America
- Brazil has implemented a national testing strategy that includes partnerships with private laboratories and universities, significantly increasing the availability of COVID-19 tests across the country.
- Countries like Chile have adopted innovative digital health solutions to streamline testing processes, with platforms that allow citizens to book tests and receive results quickly, enhancing overall public health response.
North America
- The U.S. Food and Drug Administration (FDA) has accelerated the approval process for COVID-19 diagnostic tests, allowing for more rapid deployment of innovative testing solutions, such as at-home testing kits from companies like Abbott and Quidel.
- The increasing use of telemedical services has increased the demand for remote diagnostics. Companies such as LabCorp and Quest have therefore expanded their services to include virtual consultations and home sample collection.
Middle East And Africa
- The World Health Organization (WHO) has been actively involved in supporting COVID-19 testing initiatives in Africa, providing funding and resources to enhance testing capabilities in countries like South Africa and Kenya.
- Local companies, such as BioSure in South Africa, are innovating by developing rapid testing solutions tailored to the region's unique healthcare challenges, which is expected to improve access to diagnostics in underserved areas.
Did You Know?
“As of 2023, over 1.5 billion COVID-19 diagnostic tests have been conducted globally, highlighting the critical role of testing in managing the pandemic.” — World Health Organization (WHO)
Segmental Market Size
Among the most important segments of the market is that of COVID-19 diagnostics. Its growth is currently being driven by the ever-increasing need for reliable and rapid diagnostics. The main factors driving this growth are a rising awareness of the importance of health and safety, regulatory requirements to test in different environments, and developments in diagnostic technology. The emergence of a range of new testing methods, such as the Emergency Use Authorization (EUA) procedure from the FDA, has also increased access to diagnostics. In North America and Europe, the leading diagnostics companies are Abbott Laboratories and Roche, and their products include rapid antigen and PCR tests. Their main applications are point-of-care testing in hospitals, home testing, and large-scale testing in schools and the workplace. The emergence of new COVID-19 variants and government regulations requiring testing will continue to fuel growth. The emergence of new technology, such as CRISPR-based diagnostics and data analysis using artificial intelligence, will also be important.
Future Outlook
COVID-19 Diagnostics Market to Grow Stably from 2024 to 2035. The projected market size is expected to increase from $ 7.87 billion to $ 12.0 billion, at a CAGR of 3.91%. The growth of the market is driven by the need for effective diagnostics, as the world continues to face the complexities of COVID-19 and its variants. As health care systems continue to adapt to the endemic phase of the disease, the demand for rapid, accurate, and accessible diagnostics will continue to grow, especially in regions with variable infection rates and new variants. Artificial intelligence in diagnostics and the development of point-of-care testing solutions are expected to further drive the market. In addition, supportive government policies that aim to enhance public health and increase funding for research and development in diagnostics will boost market growth. Home testing kits and digital health solutions are expected to change the behavior of consumers and increase the availability of diagnostics. The COVID-19 diagnostics landscape will continue to evolve, with a focus on innovation and flexibility to meet the ongoing challenges of the pandemic.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 6.82% (2022-2030) |
COVID 19 Diagnostics Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









