Rising Prevalence of Epilepsy
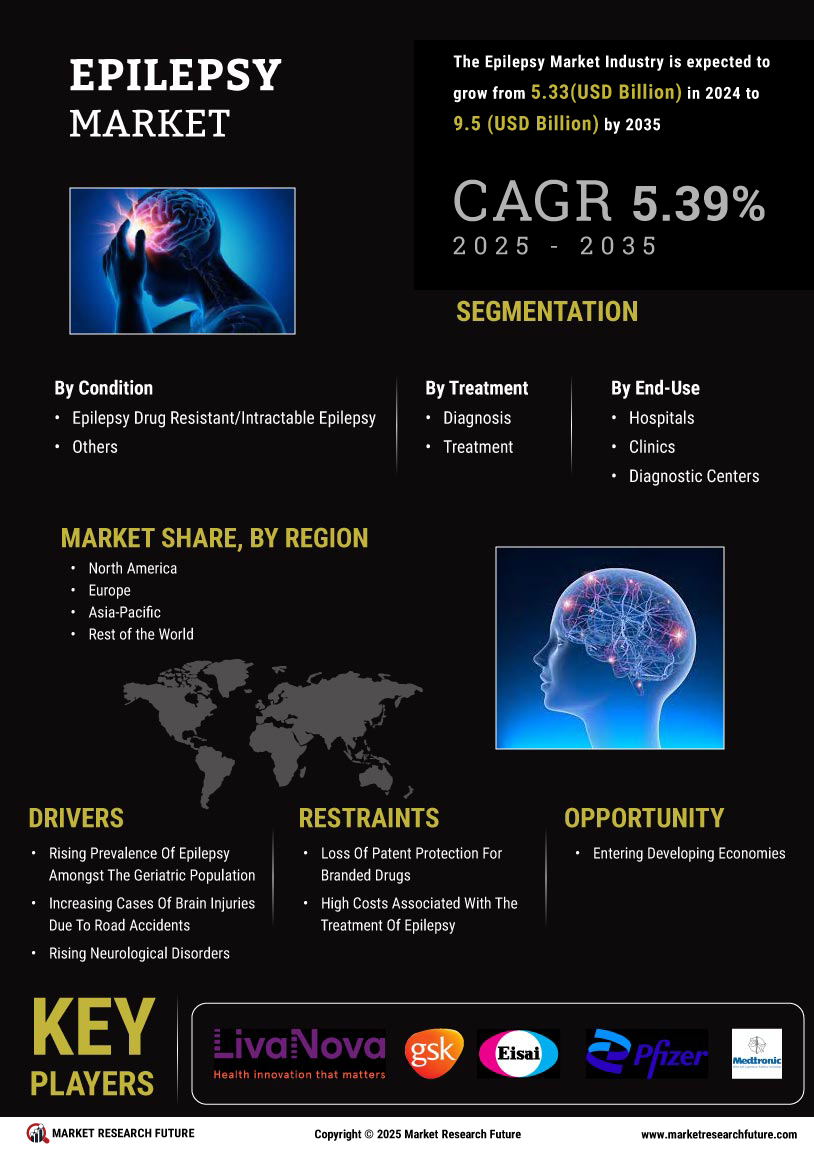
The increasing prevalence of epilepsy is a primary driver of the Epilepsy Market. Recent estimates indicate that approximately 50 million individuals worldwide are affected by epilepsy, with a notable rise in cases reported in various demographics. This surge in prevalence necessitates enhanced treatment options and healthcare resources, thereby propelling market growth. The Epilepsy Market is responding to this demand by expanding its portfolio of antiepileptic drugs and innovative therapies. Furthermore, the growing awareness of epilepsy as a chronic condition has led to increased healthcare utilization, further stimulating market dynamics. As more individuals seek diagnosis and treatment, the Epilepsy Market is likely to experience sustained growth, driven by the need for effective management solutions.
Growing Awareness and Advocacy
The rising awareness and advocacy surrounding epilepsy are pivotal in shaping the Epilepsy Market. Increased public education campaigns and initiatives led by advocacy groups have contributed to a better understanding of the condition. This heightened awareness has encouraged individuals to seek medical attention and treatment, thereby driving market demand. Furthermore, the Epilepsy Market is benefiting from collaborations between healthcare providers and advocacy organizations, which aim to improve patient outcomes and access to care. As awareness continues to grow, it is anticipated that more individuals will be diagnosed and treated, further propelling the market forward. This trend underscores the importance of education in fostering a supportive environment for those affected by epilepsy.
Advancements in Treatment Modalities
Innovations in treatment modalities are significantly influencing the Epilepsy Market. The development of new antiepileptic drugs, including novel mechanisms of action, has expanded therapeutic options for patients. For instance, the introduction of medications that target specific types of seizures has improved treatment outcomes. Additionally, advancements in surgical techniques and neurostimulation devices have provided alternative solutions for refractory epilepsy cases. The Epilepsy Market is witnessing a shift towards more personalized treatment approaches, which may enhance patient adherence and overall satisfaction. As these advancements continue to emerge, they are expected to drive market growth by offering more effective and tailored treatment options for individuals living with epilepsy.
Regulatory Support and Policy Initiatives
Regulatory support and policy initiatives play a crucial role in the development of the Epilepsy Market. Governments and health authorities are increasingly recognizing the need for comprehensive epilepsy care, leading to the implementation of supportive policies. These initiatives often include funding for research, improved access to medications, and the establishment of epilepsy centers of excellence. Such measures are designed to enhance the quality of care for individuals with epilepsy, thereby stimulating market growth. The Epilepsy Market stands to benefit from these regulatory frameworks, which may facilitate the introduction of new therapies and improve patient access to existing treatments. As policy support continues to evolve, it is likely to create a more favorable environment for innovation and market expansion.
Increased Investment in Research and Development
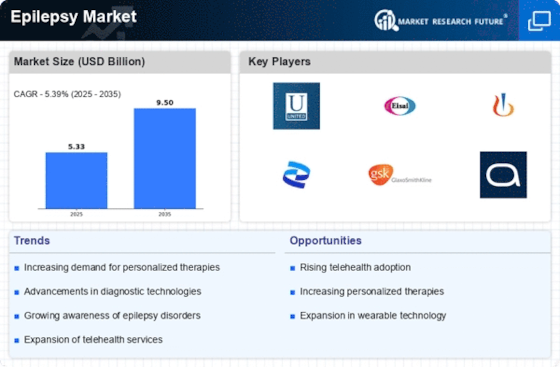
The Epilepsy Market is experiencing a surge in investment directed towards research and development initiatives. Pharmaceutical companies and research institutions are allocating substantial resources to explore novel therapies and improve existing treatment options. This focus on R&D is driven by the need to address unmet medical needs within the epilepsy community. Recent data suggests that The Epilepsy Market is projected to reach USD 7.5 billion by 2026, reflecting the potential for lucrative returns on investment. As more stakeholders recognize the importance of innovation in the Epilepsy Market, the influx of funding is likely to accelerate the development of groundbreaking therapies, ultimately benefiting patients and healthcare providers alike.