Top Industry Leaders in the Europe CRO Market

Parexel Acquired US-based Clinical Trials Services by Catalent Pharma Solutions for €1.2 billion, aiming to strengthen its global footprint and capabilities.
ICON Purchased US-based PRA Healthsciences in a $12 billion deal, solidifying its position as a leading global CRO.
BioPharma Services Holding Received a €475 million investment from the Carlyle Group to support its European expansion and clinical development services.
Syneos Health Announced a strategic partnership with the University of Oxford to establish a center for excellence in clinical research and innovation.IQVIA Launched a new AI-powered platform, IQuintain, to optimize clinical trial design and conduct.
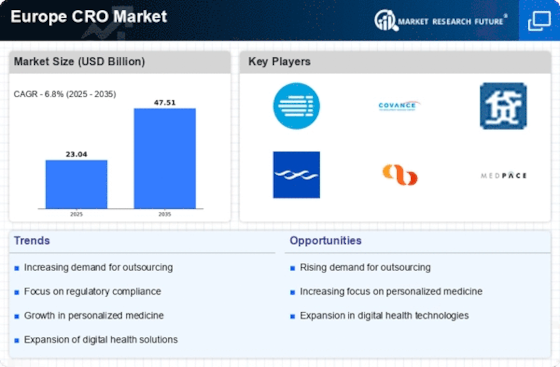
List of Europe CRO Key Companies in the Market
- Pharmaceutical Product Development, LLC (Thermo Fisher Scientific ) (U.S.)
- Medpace Holdings, Inc. (U.S.)
- Parexel International Corporation (Ireland)
- IQVIA (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Charles River Laboratories (U.S.)
- PHASTAR (U.K.)
- Oy 4Pharma Ltd (Finland)
- PSI (Switzerland)
- Icon plc (Ireland)