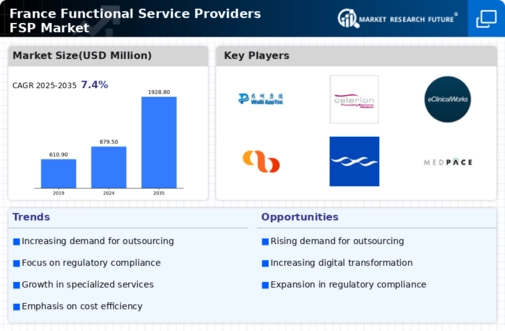
The France functional service providers market exhibits a dynamic competitive landscape characterized by a blend of innovation, strategic partnerships, and regional expansion. Key players such as IQVIA (FR), Covance (FR), and PRA Health Sciences (FR) are at the forefront, each adopting distinct strategies to enhance their market positioning. IQVIA (FR) focuses on leveraging advanced analytics and technology to optimize clinical trial processes, while Covance (FR) emphasizes its comprehensive service offerings, including drug development and commercialization solutions. PRA Health Sciences (FR) appears to be concentrating on expanding its operational footprint in Europe, thereby enhancing its service capabilities and client reach. Collectively, these strategies contribute to a competitive environment that is increasingly driven by technological advancements and client-centric solutions.
In terms of business tactics, companies are increasingly localizing their operations to better serve the French market, which may involve optimizing supply chains and enhancing service delivery. The market structure is moderately fragmented, with several players vying for market share. However, the influence of major companies is substantial, as they set benchmarks for service quality and operational efficiency, thereby shaping the competitive dynamics.
In December 2025, IQVIA (FR) announced a strategic partnership with a leading French biotechnology firm to enhance its clinical trial capabilities. This collaboration is expected to leverage both companies' strengths, potentially accelerating the development of innovative therapies. Such partnerships are indicative of a broader trend towards collaboration in the industry, which may enhance operational efficiencies and drive innovation.
In November 2025, Covance (FR) launched a new digital platform aimed at streamlining the drug development process. This initiative reflects a growing emphasis on digital transformation within the sector, as companies seek to improve data management and enhance client engagement. The strategic importance of this move lies in its potential to reduce time-to-market for new therapies, thereby providing Covance (FR) with a competitive edge.
In October 2025, PRA Health Sciences (FR) expanded its service offerings by acquiring a niche clinical research organization specializing in rare diseases. This acquisition not only broadens PRA's portfolio but also positions the company to tap into a growing market segment that requires specialized expertise. The strategic significance of this move is underscored by the increasing demand for tailored solutions in the clinical research landscape.
As of January 2026, the competitive trends in the France functional service providers market are increasingly defined by digitalization, sustainability, and the integration of artificial intelligence (AI). Strategic alliances are becoming more prevalent, as companies recognize the value of collaboration in driving innovation and enhancing service delivery. Looking ahead, competitive differentiation is likely to evolve, with a shift from price-based competition towards a focus on technological innovation, supply chain reliability, and the ability to deliver customized solutions. This transition may redefine the competitive landscape, compelling companies to invest in advanced technologies and sustainable practices to maintain their market positions.