Market Growth Projections
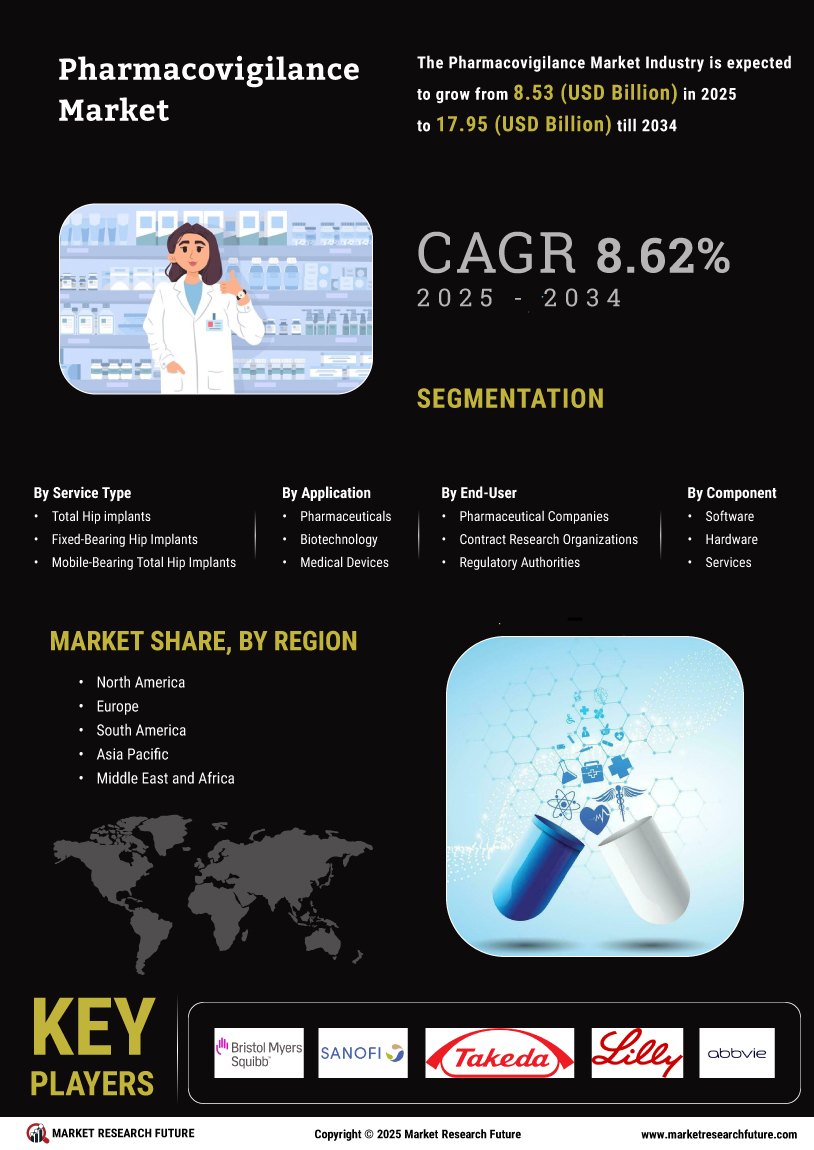
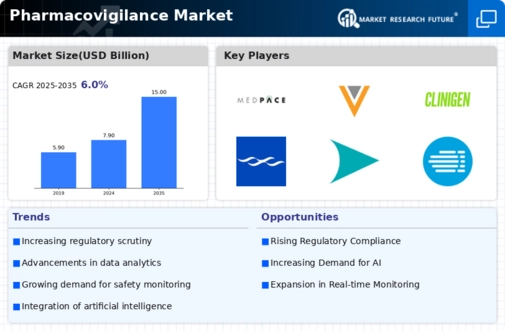
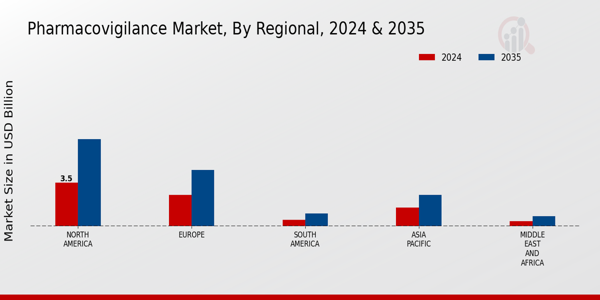
The Global Pharmacovigilance Market Industry is on a trajectory of substantial growth, with projections indicating a market size of 7.9 USD Billion in 2024 and an anticipated increase to 15 USD Billion by 2035. This growth is underpinned by a compound annual growth rate (CAGR) of 6.0% from 2025 to 2035. The expansion is driven by various factors, including increasing regulatory requirements, technological advancements, and a growing focus on patient safety. As the industry evolves, stakeholders are likely to invest in innovative pharmacovigilance solutions to enhance drug safety monitoring and compliance.
Expansion of Biopharmaceuticals
The expansion of biopharmaceuticals is reshaping the Global Pharmacovigilance Market Industry. As the biopharmaceutical sector continues to grow, the complexity of monitoring these products increases. Biologics often have unique safety profiles that require specialized pharmacovigilance approaches. Regulatory agencies are emphasizing the need for rigorous safety monitoring of biopharmaceuticals, leading to increased investments in pharmacovigilance systems. This trend is expected to drive market growth, as companies seek to ensure compliance with regulatory requirements and safeguard patient health. The market's trajectory indicates a robust future, with significant contributions from the biopharmaceutical sector.
Growing Focus on Patient Safety
The emphasis on patient safety is a significant driver of the Global Pharmacovigilance Market Industry. As healthcare stakeholders prioritize the well-being of patients, there is an increasing demand for effective monitoring of drug safety profiles. Initiatives aimed at improving patient safety, such as the establishment of patient registries and safety surveillance programs, are gaining traction. This focus is reflected in the growing investments in pharmacovigilance systems by pharmaceutical companies. The market is expected to grow at a CAGR of 6.0% from 2025 to 2035, underscoring the commitment to enhancing patient safety through rigorous pharmacovigilance practices.
Increasing Regulatory Requirements
The Global Pharmacovigilance Market Industry is experiencing heightened scrutiny from regulatory bodies worldwide. Governments and health authorities are mandating stricter reporting and monitoring of adverse drug reactions. This regulatory landscape compels pharmaceutical companies to enhance their pharmacovigilance systems, ensuring compliance and patient safety. For instance, the European Medicines Agency has implemented stringent guidelines that necessitate comprehensive risk management plans. As a result, the market is projected to reach 7.9 USD Billion in 2024, reflecting the growing need for robust pharmacovigilance frameworks to meet these evolving regulatory demands.
Rising Incidence of Adverse Drug Reactions
The rising incidence of adverse drug reactions (ADRs) is a critical factor driving the Global Pharmacovigilance Market Industry. With the increasing complexity of drug therapies and polypharmacy, the occurrence of ADRs is becoming more prevalent. This trend necessitates robust pharmacovigilance systems to monitor and manage these reactions effectively. For instance, reports indicate that ADRs account for a significant percentage of hospital admissions, highlighting the urgent need for improved safety monitoring. Consequently, the market is poised for growth as healthcare providers and pharmaceutical companies invest in comprehensive pharmacovigilance strategies to mitigate the risks associated with ADRs.
Technological Advancements in Data Management
Technological innovations are transforming the Global Pharmacovigilance Market Industry, particularly in data management and analysis. Advanced software solutions and artificial intelligence are being integrated into pharmacovigilance processes, enabling real-time monitoring of drug safety. These technologies facilitate the efficient collection, analysis, and reporting of adverse events, thereby improving decision-making. For example, machine learning algorithms can identify potential safety signals from vast datasets, enhancing the overall effectiveness of pharmacovigilance. This trend is likely to contribute to the market's growth, with projections indicating a rise to 15 USD Billion by 2035, driven by the demand for more sophisticated data management tools.