Market Growth Projections
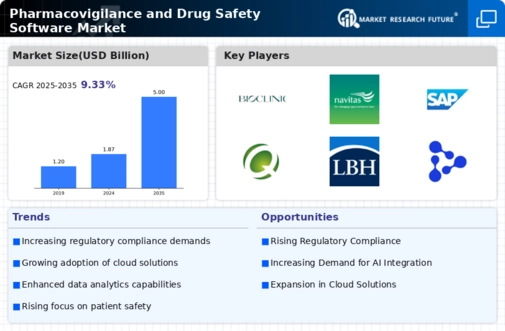
The Global Pharmacovigilance and Drug Safety Software Market Industry is poised for substantial growth, with projections indicating a market value of 1.87 USD Billion in 2024 and an anticipated increase to 5 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate (CAGR) of 9.35% from 2025 to 2035. Such projections highlight the increasing recognition of the importance of pharmacovigilance in ensuring drug safety and efficacy. As regulatory bodies continue to emphasize the need for robust monitoring systems, the market is likely to expand, driven by technological advancements and the growing demand for comprehensive drug safety solutions.
Rising Incidence of Adverse Drug Reactions
The Global Pharmacovigilance and Drug Safety Software Market Industry is propelled by the rising incidence of adverse drug reactions (ADRs). With an increasing number of drugs entering the market, the potential for ADRs escalates, prompting healthcare providers and pharmaceutical companies to prioritize drug safety. Reports indicate that ADRs contribute significantly to hospital admissions and healthcare costs, underscoring the need for robust pharmacovigilance systems. The industry's growth trajectory is evident, as it is projected to reach 5 USD Billion by 2035, driven by the necessity for effective monitoring and reporting of ADRs to safeguard patient health and enhance therapeutic outcomes.
Growing Demand for Patient-Centric Approaches
The Global Pharmacovigilance and Drug Safety Software Market Industry is increasingly influenced by the growing demand for patient-centric approaches in healthcare. Patients are becoming more engaged in their treatment processes, leading to a heightened focus on drug safety and efficacy. This shift necessitates the implementation of software solutions that facilitate patient feedback and adverse event reporting. By integrating patient perspectives into pharmacovigilance practices, pharmaceutical companies can enhance their safety profiles and foster trust among consumers. The industry's evolution towards patient-centricity is indicative of a broader trend in healthcare, aligning with the increasing emphasis on personalized medicine and patient empowerment.
Increasing Regulatory Compliance Requirements
The Global Pharmacovigilance and Drug Safety Software Market Industry experiences a surge in demand due to escalating regulatory compliance requirements. Regulatory bodies worldwide, such as the FDA and EMA, enforce stringent guidelines for drug safety monitoring. This necessitates the adoption of advanced software solutions to ensure compliance and mitigate risks associated with adverse drug reactions. As of 2024, the market is valued at approximately 1.87 USD Billion, reflecting the industry's responsiveness to regulatory pressures. Companies are increasingly investing in pharmacovigilance systems to streamline reporting processes and enhance data accuracy, thereby fostering a culture of safety and accountability in drug development.
Technological Advancements in Data Management
Technological advancements in data management are transforming the Global Pharmacovigilance and Drug Safety Software Market Industry. Innovations such as artificial intelligence and machine learning facilitate the analysis of vast datasets, enabling quicker identification of safety signals and trends. These technologies enhance the efficiency of pharmacovigilance processes, allowing for real-time monitoring and reporting of adverse events. As organizations seek to leverage these advancements, the market is expected to grow at a compound annual growth rate (CAGR) of 9.35% from 2025 to 2035. This growth reflects a broader trend towards data-driven decision-making in drug safety, ultimately improving patient outcomes and regulatory compliance.
Expansion of Biopharmaceuticals and Personalized Medicine
The expansion of biopharmaceuticals and personalized medicine significantly impacts the Global Pharmacovigilance and Drug Safety Software Market Industry. As the biopharmaceutical sector grows, the complexity of drug safety monitoring increases, necessitating sophisticated software solutions to manage diverse data sources and regulatory requirements. The rise of personalized medicine, which tailors treatments to individual patient profiles, further complicates pharmacovigilance efforts. Companies must adopt advanced software to ensure comprehensive safety monitoring and compliance with evolving regulations. This trend is expected to drive substantial growth in the market, reflecting the industry's adaptability to the changing landscape of drug development and patient care.