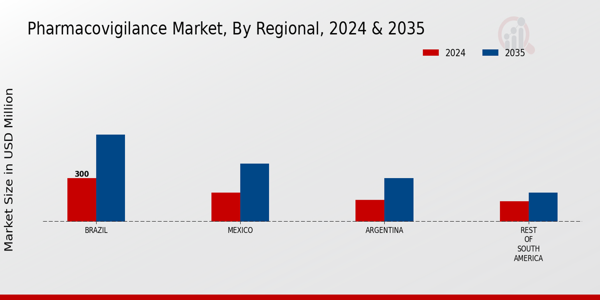
The South America Pharmacovigilance Market is characterized by a landscape that is steadily evolving, influenced by stringent regulatory requirements, increasing demand for patient safety, and the growing complexity of drug development processes. As pharmaceutical companies seek to enhance their medication monitoring systems, the competitive environment is now marked by innovative solutions, strategic collaborations, and a focus on technology-driven advancements.
Companies operating in this market are leveraging data analytics, artificial intelligence, and real-time monitoring to enhance their pharmacovigilance capabilities, ensuring compliance with regulatory mandates while addressing the challenges posed by drug safety. This competitive domain is witnessing heightened activity as firms vie for market share, catering to the region's unique healthcare challenges and regulatory conditions, thereby creating a dynamic ecosystem for pharmacovigilance services.Within the South America Pharmacovigilance Market, Parexel International stands out as a leading player known for its robust service offerings and commitment to patient safety.
The company boasts a strong presence across Brazil and other key countries in the region, where it provides comprehensive pharmacovigilance solutions tailored to meet local regulatory requirements. Parexel International's strengths lie in its extensive experience in clinical development and regulatory consulting, allowing it to effectively support pharmaceutical and biotechnology companies in navigating the complexities of drug safety evaluation.
The firm’s integration of cutting-edge technology aids in streamlining adverse event reporting and ensures efficient signal detection, thereby reinforcing its position as a trusted partner in the pharmacovigilance arena of South America.Syntropy, operating in the South America Pharmacovigilance Market, focuses on delivering innovative pharmacovigilance and risk management solutions.
The company is recognized for its commitment to utilizing advanced technologies to enhance data analysis and improve the quality of safety reporting. Syntropy's portfolio includes a range of services, such as post-marketing surveillance, data mining, and regulatory compliance services, which are crucial in addressing the diverse needs of pharmaceutical clients in the region. The firm has made significant strides in establishing its market presence through strategic partnerships and collaborations with local healthcare authorities and institutions.
Additionally, Syntropy's strengths include a dedicated focus on building robust safety databases and a commitment to continuous improvement through mergers and acquisitions that allow for the expansion of its service capabilities. This focus has enabled Syntropy to reinforce its standing in the South America pharmacovigilance landscape, ensuring that it remains competitive and responsive to the evolving demands of the market.