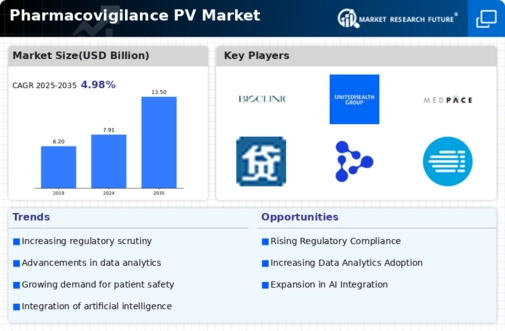
The global pharmacovigilance market is influenced by several key drivers that shape its growth trajectory. The increasing focus on patient safety and regulatory compliance across the healthcare sector is a significant catalyst. Pharmaceutical companies are becoming more vigilant in monitoring the safety of their products, leading to a heightened demand for effective pharmacovigilance solutions.
Additionally, the rise in adverse drug reactions and the increasing need for post-marketing surveillance contribute to the growing market. The expanding pipeline of new drugs, along with the complexities surrounding drug approvals, further necessitates robust PV systems to ensure adherence to safety regulations.
There are numerous opportunities within the pharmacovigilance landscape that can be explored. With advancements in technology, the integration of artificial intelligence and machine learning into pharmacovigilance processes can enhance data analysis and reporting efficiency.
The global expansion of the pharmaceutical market also offers new avenues for collaboration, tapping into emerging markets that may require tailored pharmacovigilance strategies.In recent times, the pharmacovigilance industry has seen an increased emphasis on real-world evidence, with organizations seeking data beyond clinical trials.
This shift highlights the need for continuous monitoring of drug effectiveness and safety in diverse populations. Additionally, regulatory agencies worldwide are updating guidelines, making it essential for companies to adopt agile pharmacovigilance practices.
The importance of data transparency and communication among stakeholders has also gained momentum, promoting a more collaborative approach to drug safety. Overall, the pharmacovigilance market is undergoing a dynamic evolution, shaped by these trends and opportunities, emphasizing the need for awareness and adaptation in a rapidly changing environment.
Key Pharmacovigilance PV Market Trends Highlighted
The global pharmacovigilance market is influenced by several key drivers that shape its growth trajectory. The increasing focus on patient safety and regulatory compliance across the healthcare sector is a significant catalyst. Pharmaceutical companies are becoming more vigilant in monitoring the safety of their products, leading to a heightened demand for effective pharmacovigilance solutions.
Additionally, the rise in adverse drug reactions and the increasing need for post-marketing surveillance contribute to the growing market. The expanding pipeline of new drugs, along with the complexities surrounding drug approvals, further necessitates robust PV systems to ensure adherence to safety regulations.
There are numerous opportunities within the pharmacovigilance landscape that can be explored. With advancements in technology, the integration of artificial intelligence and machine learning into pharmacovigilance processes can enhance data analysis and reporting efficiency.
The global expansion of the pharmaceutical market also offers new avenues for collaboration, tapping into emerging markets that may require tailored pharmacovigilance strategies.In recent times, the pharmacovigilance industry has seen an increased emphasis on real-world evidence, with organizations seeking data beyond clinical trials.
This shift highlights the need for continuous monitoring of drug effectiveness and safety in diverse populations. Additionally, regulatory agencies worldwide are updating guidelines, making it essential for companies to adopt agile pharmacovigilance practices.
The importance of data transparency and communication among stakeholders has also gained momentum, promoting a more collaborative approach to drug safety. Overall, the pharmacovigilance market is undergoing a dynamic evolution, shaped by these trends and opportunities, emphasizing the need for awareness and adaptation in a rapidly changing environment.
The increasing complexity of drug development and regulatory requirements appears to drive the demand for robust pharmacovigilance systems, as stakeholders seek to ensure patient safety and compliance with evolving standards.
U.S. Food and Drug Administration (FDA)