-
Report Prologue
-
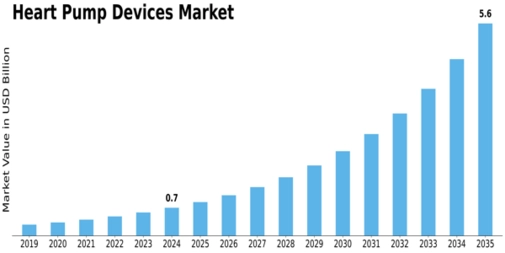
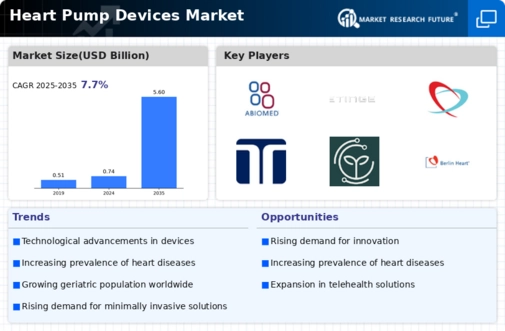
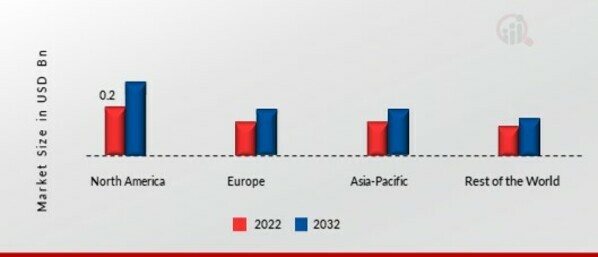
Market Introduction
-
Definition
-
Scope Of The Study
- Research Objective
- Limitations
-
Assumptions
-
Research Methodology
-
Introduction
-
Primary Research
-
Secondary Research
-
Market Size Estimation
-
Market Dynamics
-
Drivers
-
Restraints
-
Opportunities
-
Challenges
-
Macroeconomic Indicators
-
Technology Trends &
-
Assessment
-
Disease Prevalence And Diagnosis Analysis, By Country
-
Market Factor Analysis
-
Porter’s Five Forces Analysis
- Bargaining
- Bargaining Power Of Buyers
- Threat Of New
- Threat Of Substitutes
- Intensity Of Rivalry
-
Power Of Suppliers
-
Entrants
-
Value Chain Analysis
-
Investment Feasibility Analysis
-
Pricing Analysis
-
Global Heart Pump Devices Market, By Product
-
Introduction
-
Ventricular Assist Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Left Ventricular Assist Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Right Ventricular Assist Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Bi-Ventricular Assist Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Percutaneous Ventricular Assist Devices
-
• Market Estimates & Forecast,
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
• Market Estimates &
-
Forecast, 2023-2032
-
Transcutaneous Ventricular Assist Devices
-
• Market Estimates & Forecast, By Region/Country,
-
Intra-Aortic Balloon Pumps
-
• Market Estimates &
-
Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country,
-
Total Artificial Heart
-
• Market Estimates & Forecast,
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Global Heart Pump Devices Market, By Device Type
-
Introduction
-
Implantable Heart Pump Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Extracorporeal Heart Pump Devices
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Global
-
Heart Pump Devices Market, By Therapy
-
Introduction
-
Bridge-To-Candidacy
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates
-
& Forecast, By Region/Country, 2023-2032
-
Bridge-To-Transplant
-
•
-
Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast,
-
By Region/Country, 2023-2032
-
Destination Therapy
-
• Market Estimates
-
& Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country,
-
Others
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
•
-
Global Heart Pump Devices Market, By End-User
-
Introduction
-
Hospitals & Clinics
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Ambulatory Surgical Centers
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates & Forecast, By Region/Country, 2023-2032
-
Research Institutes
-
• Market Estimates & Forecast, 2023-2032
-
•
-
Market Estimates & Forecast, By Region/Country, 2023-2032
-
Others
-
• Market Estimates & Forecast, 2023-2032
-
• Market Estimates
-
& Forecast, By Region/Country, 2023-2032
-
Global Heart Pump Devices Market,
-
By Region
-
Introduction
-
Americas
-
• Market Estimates
-
& Forecast, By Region, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
•
-
North America
-
Market Estimates & Forecast, By Country, 2023-2032
-
• Market Estimates
-
& Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast,
-
By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
U.S.
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
Canada
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
•
-
South America
-
Market Estimates & Forecast, By Product, 2023-2032
-
• Market Estimates
-
& Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast,
-
By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User,
-
Europe
-
• Market Estimates & Forecast, By Region,
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market
-
Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates &
-
Forecast, By End-User, 2023-2032
-
• Market Estimates
-
& Forecast, By Country, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
Western Europe
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
•
-
Germany
-
Market Estimates & Forecast, By Product, 2023-2032
-
• Market Estimates
-
& Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast,
-
By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User,
-
France
-
• Market Estimates & Forecast, By Product,
-
• Market Estimates & Forecast, By Type, 2023-2032
-
•
-
Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates
-
& Forecast, By End-User, 2023-2032
-
• Market Estimates
-
& Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast,
-
By Type, 2023-2032
-
U.K.
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
Italy
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
Spain
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
Rest Of Western Europe
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates &
-
Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast, By
-
Type, 2023-2032
-
Eastern Europe
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
Asia
-
Pacific
-
• Market Estimates & Forecast, By Country, 2023-2032
-
•
-
Market Estimates & Forecast, By Product, 2023-2032
-
• Market Estimates
-
& Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast,
-
By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User,
-
Japan
-
• Market Estimates & Forecast, By Product,
-
• Market Estimates & Forecast, By Type, 2023-2032
-
•
-
Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates
-
& Forecast, By End-User, 2023-2032
-
• Market Estimates
-
& Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast,
-
By Type, 2023-2032
-
China
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
India
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
Australia
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
Republic Of Korea
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates
-
& Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast,
-
By Type, 2023-2032
-
Rest Of Asia Pacific
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
The Middle
-
East & Africa
-
• Market Estimates & Forecast, By Region, 2023-2032
-
• Market Estimates & Forecast, By Product, 2023-2032
-
• Market
-
Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates &
-
Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By
-
End-User, 2023-2032
-
• Market Estimates
-
& Forecast, By Product, 2023-2032
-
• Market Estimates & Forecast,
-
By Type, 2023-2032
-
United Arab Emirates
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User, 2023-2032
-
Arabia
-
Saudi
-
• Market Estimates & Forecast, By Product, 2023-2032
-
•
-
Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates
-
& Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast,
-
By End-User, 2023-2032
-
• Market Estimates & Forecast,
-
By Product, 2023-2032
-
Oman
-
• Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market
-
Estimates & Forecast, By End-User, 2023-2032
-
•
-
Kuwait
-
Market Estimates & Forecast, By Product, 2023-2032
-
• Market Estimates
-
& Forecast, By Type, 2023-2032
-
• Market Estimates & Forecast,
-
By Therapy, 2023-2032
-
• Market Estimates & Forecast, By End-User,
-
Qatar
-
• Market Estimates & Forecast, By Product,
-
• Market Estimates & Forecast, By Type, 2023-2032
-
•
-
Market Estimates & Forecast, By Therapy, 2023-2032
-
• Market Estimates
-
& Forecast, By End-User, 2023-2032
-
Africa
-
Rest Of The Middle East &
-
• Market Estimates & Forecast, By Product, 2023-2032
-
•
-
Market Estimates & Forecast, By Type, 2023-2032
-
• Market Estimates
-
& Forecast, By Therapy, 2023-2032
-
• Market Estimates & Forecast,
-
By End-User, 2023-2032
-
Company Landscape
-
Introduction
-
Market Share Analysis
-
Key Development & Strategies
- Key
-
Developments
-
Company Profiles
-
Abbott Laboratories
- Product Overview
- Financials
-
Company Overview
-
SWOT Analysis
-
Abiomed
- Company Overview
- Product
- Financial Overview
- Key Developments
-
Overview
-
SWOT Analysis
-
Medtronic
- Company Overview
- Product
- Financial Overview
- Key Development
-
Overview
-
SWOT Analysis
-
Getinge
- Company Overview
- Product/Business
- Financial Overview
- Key Development
-
Segment Overview
-
SWOT Analysis
-
Syncardia Systems
- Company Overview
- Financial Overview
- Key Developments
-
Product Overview
-
Teleflex
- Company Overview
- Product Overview
- Key Developments
-
Financial Overview
-
Reliantheart
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Overview
-
Terumo
- Overview
- Product
- Financials
- Key Developments
- SWOT Analysis
-
Overview
-
Berlin Heart
- Overview
- Product Overview
- Key Developments
- SWOT Analysis
-
Financials
-
Jarvik
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Heart
-
Cardiacassist
- Overview
- Product Overview
- Financials
- SWOT Analysis
-
Key Developments
-
Fresenius Medical Care
- Overview
- Product Overview
- Financials
- SWOT Analysis
-
Key Developments
-
Thoratec Corporation
- Product Overview
- Financials
- Key
- SWOT Analysis
-
Overview
-
Developments
-
HeartWare
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
MAQUET GmbH & Co
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Appendix
-
LIST OF TABLES
-
Heart
-
Pump Devices Industry Synopsis, 2023-2032
-
Global Heart Pump Devices
-
Market Estimates And Forecast, 2023-2032, (USD Million)
-
Global Heart
-
Pump Devices Market, By Region, 2023-2032, (USD Million)
-
Global Heart
-
Pump Devices Market, By Type, 2023-2032, (USD Million)
-
Global Heart
-
Pump Devices Market, By Product, 2023-2032, (USD Million)
-
Global Heart
-
Pump Devices Market, By Therapy, 2023-2032, (USD Million)
-
Global Heart
-
Pump Devices Market, By End-Users, 2023-2032, (USD Million)
-
North America
-
Heart Pump Devices Market, By Type, 2023-2032, (USD Million)
-
North
-
America Heart Pump Devices Market, By Therapy, 2023-2032, (USD Million)
-
Table
-
North America Heart Pump Devices Market, By Product, 2023-2032, (USD Million)
-
North America Heart Pump Devices Market, By End-Users, 2023-2032, (USD
-
Million)
-
U.S. Heart Pump Devices Market, By Type, 2023-2032, (USD
-
Million)
-
U.S. Heart Pump Devices Market, By Therapy, 2023-2032, (USD
-
Million)
-
U.S. Heart Pump Devices Market, By Product, 2023-2032, (USD
-
Million)
-
U.S. Heart Pump Devices Market, By End-Users, 2023-2032,
-
(USD Million)
-
Canada Heart Pump Devices Market, By Type, 2023-2032,
-
(USD Million)
-
Canada Heart Pump Devices Market, By Product, 2023-2032,
-
(USD Million)
-
Canada Heart Pump Devices Market, By Therapy, 2023-2032,
-
(USD Million)
-
Canada Heart Pump Devices Market, By End-Users, 2023-2032,
-
(USD Million)
-
South America Heart Pump Devices Market, By Type, 2023-2032,
-
(USD Million)
-
South America Heart Pump Devices Market, By Therapy,
-
South America Heart Pump Devices Market,
-
By End-Users, 2023-2032, (USD Million)
-
Europe Heart Pump Devices Market,
-
By Type, 2023-2032, (USD Million)
-
Europe Heart Pump Devices Market,
-
By Therapy, 2023-2032, (USD Million)
-
Europe Heart Pump Devices Market,
-
By End-Users, 2023-2032, (USD Million)
-
Western Europe Heart Pump Devices
-
Market, By Type, 2023-2032, (USD Million)
-
Western Europe Heart Pump
-
Devices Market, By Therapy, 2023-2032, (USD Million)
-
Western Europe
-
Heart Pump Devices Market, By End-Users, 2023-2032, (USD Million)
-
Table 26
-
Eastern Europe Heart Pump Devices Market, By Type, 2023-2032, (USD Million)
-
Table
-
Eastern Europe Heart Pump Devices Market, By Therapy, 2023-2032, (USD Million)
-
Eastern Europe Heart Pump Devices Market, By End-Users, 2023-2032, (USD
-
Million)
-
Asia Pacific Heart Pump Devices Market, By Type, 2023-2032,
-
(USD Million)
-
Asia Pacific Heart Pump Devices Market, By Therapy,
-
Asia Pacific Heart Pump Devices Market, By
-
End-Users, 2023-2032, (USD Million)
-
Middle East & Africa Heart
-
Pump Devices Market, By Type, 2023-2032, (USD Million)
-
Middle East
-
& Africa Heart Pump Devices Market, By Diagnoses, 2023-2032, (USD Million)
-
Middle East & Africa Heart Pump Devices Market, By End-Users, 2023-2032,
-
(USD Million)
-
-
LIST OF FIGURES
-
Research Process
-
Segmentation For Global Heart Pump Devices Market
-
Segmentation
-
Market Dynamics For Heart Pump Devices Market
-
Global Heart Pump Devices
-
Market Share, By Type 2020
-
Global Heart Pump Devices Market Share,
-
By Product 2020
-
Global Heart Pump Devices Market Share, By End-Users,
-
Global Heart Pump Devices Market Share, By Region, 2020
-
Figure
-
North America Heart Pump Devices Market Share, By Country, 2020
-
Figure 9
-
Europe Heart Pump Devices Market Share, By Country, 2020
-
Asia Pacific
-
Heart Pump Devices Market Share, By Country, 2020
-
Middle East &
-
Africa Heart Pump Devices Market Share, By Country, 2020
-
Global Heart
-
Pump Devices Market: Company Share Analysis, 2020 (%)
-
Abbott Laboratories:
-
Key Financials
-
Abbott Laboratories: Segmental Revenue
-
Figure
-
Abbott Laboratories: Geographical Revenue
-
Medtronic. Key Financials
-
Medtronic: Segmental Revenue
-
Medtronic: Geographical
-
Revenue
-
Terumo: Key Financials
-
Terumo: Segmental Revenue
-
Terumo: Geographical Revenue
-
Getinge: Key Financials
-
Getinge: Segmental Revenue
-
Getinge: Geographical Revenue
-
Teleflex: Key Financials
-
Teleflex: Segmental Revenue
-
Teleflex. Geographical Revenue
-
Abiomed. Key Financials
-
Abiomed. Segmental Revenue
-
Abiomed. Geographical Revenue
-
Cardiacassist. Key Financials
-
Cardiacassist. Segmental
-
Revenue
-
Cardiacassist. Geographical Revenue

















Leave a Comment