-
MARKET INTRODUCTION
-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Primary Research
-
Secondary Research
-
Market Size Estimation
-
MARKET DYNAMICS
-
Overview
-
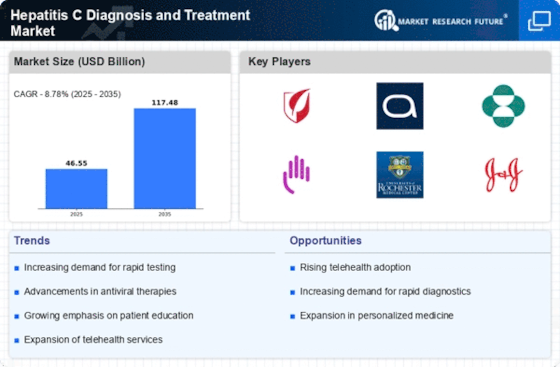
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Value Chain Analysis
-
-
Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS
-
Overview
-
Liver Function Tests
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Liver Biopsy
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Blood Tests
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Others
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Hepatitis C Diagnosis Treatment Market, BY TREATMENT
-
Overview
-
Antiviral Medications
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Immuno-Modulators
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Liver Transplantation
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Others
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Hepatitis C Diagnosis Treatment Market, BY END USER
-
Overview
-
Hospitals and Clinics
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Diagnostic Centers
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Others
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Region, 2020-2027
-
Market Estimates & Hepatitis C Diagnosis Treatment Market, by Country, 2020-2027
-
Hepatitis C Diagnosis Treatment Market, BY REGION
-
Overview
-
Americas
- North America
- Latin America
-
Europe
- Western Europe
- Eastern Europe
-
Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
-
Middle East & Africa
- Middle East
- Africa
-
COMPANY LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market Share Analysis
-
Major Growth Strategy in the Global Hepatitis C Diagnosis and Treatment Market
-
Competitive Benchmarking
-
Leading Players in Terms of the Number of Developments in the Global Hepatitis C Diagnosis and Treatment Market
-
Key Developments and Growth Strategies
- Product Launch/Service Deployment
- Mergers and Acquisitions
- Joint Ventures
-
Major Players Financial Matrix & Market Ratio
- Sales & Operating Income 2020
- Major Players R&D Expenditure 2020
-
Major Players Capital Market Ratio
-
COMPANY PROFILES
-
F. Hoffmann-La Roche Ltd
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Vertex Pharmaceuticals Incorporated
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Gilead Sciences, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
AbbVie Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
GlaxoSmithKline plc
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Novartis Pharmaceuticals Corporation
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Bristol-Myers Squibb Company
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Abbott.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Beckman Coulter, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Siemens Medical Solutions USA, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
MedMira Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
DiaSorin SpA
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Qiagen
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
bioMérieux SA
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Hologic, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Bio-Rad Laboratories, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Others
-
APPENDIX
-
References
-
Related Reports
-
-
LIST OF TABLES
-
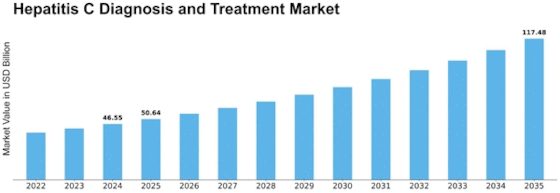
GLOBAL HEPATITIS C DIAGNOSIS AND TREATMENT MARKET SYNOPSIS, 2020-2027
-
GLOBAL HEPATITIS C DIAGNOSIS AND TREATMENT MARKET ESTIMATES & FORECAST, 2020-2027 (USD MILLION)
-
Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
Hepatitis C Diagnosis Treatment Market, BY REGION, 2020-2027 (USD MILLION)
-
NORTH AMERICA: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
NORTH AMERICA: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
NORTH AMERICA: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
US: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
US: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
US: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
CANADA: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
CANADA: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
CANADA: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
LATIN AMERICA: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
LATIN AMERICA: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
LATIN AMERICA: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
EUROPE: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
EUROPE: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
EUROPE: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
WESTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
WESTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
WESTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
EASTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
EASTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
EASTERN EUROPE: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
ASIA-PACIFIC: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
ASIA-PACIFIC: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
ASIA-PACIFIC: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020-2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: Hepatitis C Diagnosis Treatment Market, BY TREATMENT, 2020-2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: Hepatitis C Diagnosis Treatment Market, BY END USER, 2020-2027 (USD MILLION)
-
-
LIST OF FIGURES
-
RESEARCH PROCESS
-
MARKET STRUCTURE FOR THE GLOBAL HEPATITIS C DIAGNOSIS AND TREATMENT MARKET
-
MARKET DYNAMICS FOR THE GLOBAL HEPATITIS C DIAGNOSIS AND TREATMENT MARKET
-
Hepatitis C Diagnosis Treatment Market, BY DIAGNOSIS, 2020 (%)
-
Hepatitis C Diagnosis Treatment Market, BY TREATMENT TYPE, 2020 (%)
-
Hepatitis C Diagnosis Treatment Market, BY END USER, 2020 (%)
-
Hepatitis C Diagnosis Treatment Market, BY REGION, 2020 (%)
-
AMERICAS: HEPATITIS C DIAGNOSIS AND TREATMENT MARKET SHARE BY REGION, 2020 (%)
-
NORTH AMERICA: HEPATITIS C DIAGNOSIS AND TREATMENT MARKET SHARE, BY COUNTRY, 2020 (%)
-
EUROPE: Hepatitis C Diagnosis Treatment Market, BY REGION, 2020 (%)
-
WESTERN EUROPE: HEPATITIS C DIAGNOSIS AND TREATMENT MARKET SHARE, BY COUNTRY, 2020 (%)
-
ASIA-PACIFIC: Hepatitis C Diagnosis Treatment Market, BY COUNTRY, 2020 (%)
-
MIDDLE EAST & AFRICA: HEPATITIS C DIAGNOSIS AND TREATMENT MARKET SHARE, BY COUNTRY, 2020 (%)
-
GLOBAL HEPATITIS C DIAGNOSIS AND TREATMENT MARKET: COMPANY SHARE ANALYSIS, 2020 (%)
-
F. HOFFMANN-LA ROCHE LTD.: KEY FINANCIALS
-
F. HOFFMANN-LA ROCHE LTD: SEGMENTAL REVENUE
-
F. HOFFMANN-LA ROCHE LTD: REGIONAL REVENUE
-
VERTEX PHARMACEUTICALS INCORPORATED: KEY FINANCIALS
-
VERTEX PHARMACEUTICALS INCORPORATED: SEGMENTAL REVENUE
-
VERTEX PHARMACEUTICALS INCORPORATED: REGIONAL REVENUE
-
GILEAD SCIENCES, INC.: KEY FINANCIALS
-
GILEAD SCIENCES, INC.: SEGMENTAL REVENUE
-
GILEAD SCIENCES, INC.: REGIONAL REVENUE
-
ABBVIE INC.: KEY FINANCIALS
-
ABBVIE INC.: SEGMENTAL REVENUE
-
ABBVIE INC.: REGIONAL REVENUE
-
GLAXOSMITHKLINE PLC: KEY FINANCIALS
-
GLAXOSMITHKLINE PLC: SEGMENTAL REVENUE
-
GLAXOSMITHKLINE PLC: REGIONAL REVENUE
-
NOVARTIS PHARMACEUTICALS CORPORATION: KEY FINANCIALS
-
NOVARTIS PHARMACEUTICALS CORPORATION: SEGMENTAL REVENUE
-
NOVARTIS PHARMACEUTICALS CORPORATION: REGIONAL REVENUE
-
BRISTOL-MYERS SQUIBB COMPANY: KEY FINANCIALS
-
BRISTOL-MYERS SQUIBB COMPANY: SEGMENTAL REVENUE
-
BRISTOL-MYERS SQUIBB COMPANY: REGIONAL REVENUE
-
ABBOTT.: KEY FINANCIALS
-
ABBOTT.: SEGMENTAL REVENUE
-
ABBOTT.: REGIONAL REVENUE
-
BECKMAN COULTER, INC.: KEY FINANCIALS
-
BECKMAN COULTER, INC.: SEGMENTAL REVENUE
-
BECKMAN COULTER, INC.: REGIONAL REVENUE
-
SIEMENS MEDICAL SOLUTIONS USA, INC.: KEY FINANCIALS
-
SIEMENS MEDICAL SOLUTIONS USA, INC.: SEGMENTAL REVENUE
-
SIEMENS MEDICAL SOLUTIONS USA, INC.: REGIONAL REVENUE
-
MEDMIRA INC.: KEY FINANCIALS
-
MEDMIRA INC.: SEGMENTAL REVENUE
-
MEDMIRA INC.: REGIONAL REVENUE
-
DIASORIN S.P.A.: KEY FINANCIALS
-
DIASORIN S.P.A.: SEGMENTAL REVENUE
-
DIASORIN S.P.A.: REGIONAL REVENUE
-
QIAGEN: KEY FINANCIALS
-
QIAGEN: SEGMENTAL REVENUE
-
QIAGEN: REGIONAL REVENUE
-
BIOMÉRIEUX SA: KEY FINANCIALS
-
BIOMÉRIEUX SA: SEGMENTAL REVENUE
-
BIOMÉRIEUX SA: REGIONAL REVENUE
-
HOLOGIC, INC.: KEY FINANCIALS
-
HOLOGIC, INC.: SEGMENTAL REVENUE
-
HOLOGIC, INC.: REGIONAL REVENUE
-
BIO-RAD LABORATORIES, INC.: KEY FINANCIALS
-
BIO-RAD LABORATORIES, INC.: SEGMENTAL REVENUE
-
BIO-RAD LABORATORIES, INC.: REGIONAL REVENUE

















Leave a Comment