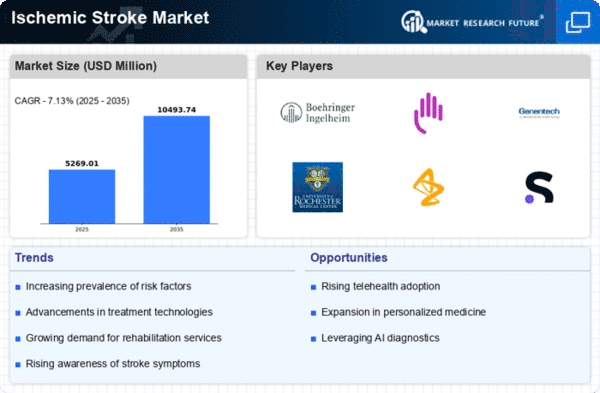
Top Industry Leaders in the Ischemic Stroke Market

Latest Ischemic Stroke Companies Update:
Royal Philips Showcases Latest Technologies at Stroke Conferences: In October 2023, Philips presented advanced imaging solutions and robotic platforms designed to streamline diagnosis and treatment during the crucial "golden hour" after an ischemic stroke
Stryker's Trevo™ Retriever Earns European Approval for Large Vessel Blockages: This January 2024 milestone expands access to a potentially life-saving mechanical thrombectomy device across Europe for patients with complex clots.
Positive Phase 2 Results for AstraZeneca's ASP7378: This December 2023 study suggests the potential of ASP7378 for protecting brain tissue after an ischemic stroke, paving the way for further research and development.
Regen Therapeutics Initiates Phase 3 Trial for Stroke Recovery Stem Cell Therapy: This January 2024 development marks a significant step towards potentially improving functional outcomes in stroke patients through regenerative medicine.
Brainomix Secures FDA Marketing Authorization for e-Stroke Score: This November 2023 approval enables healthcare professionals to utilize this AI-powered platform for rapid stroke diagnosis and treatment decision support.
List of Ischemic Stroke Key companies in the market
- GlaxoSmithKline plc (U.K)
- Eli Lilly and Company (U.S.)
- Pfizer Inc. (U.S.)
- Bristol-Myers Squibb (U.S.)
- Switzerland) Inc. (U.S.)
- Johnson & Johnson Services Inc. (U.S.)