Growing Focus on Data Security
Data security has emerged as a critical concern within the eclinical solution market in Italy. With the increasing digitization of clinical data, stakeholders are prioritizing the implementation of robust security measures to protect sensitive information. Regulatory frameworks, such as the General Data Protection Regulation (GDPR), mandate stringent data protection protocols, compelling eclinical solution providers to enhance their security offerings. As a result, the market is witnessing a shift towards solutions that incorporate advanced encryption and data management technologies. This focus on security is likely to drive market growth, as organizations seek to ensure compliance and build trust with patients and regulatory bodies.
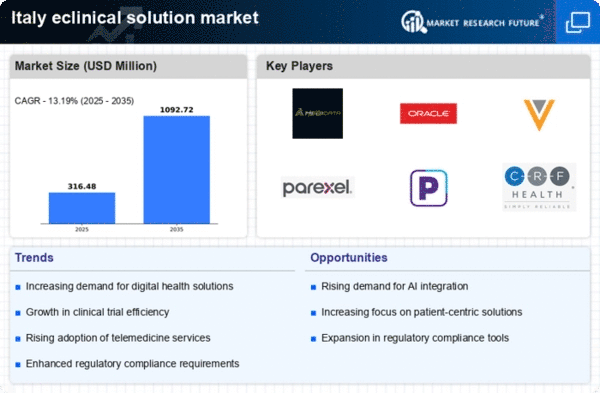
Rising Demand for Clinical Trials
The eclinical solution market in Italy is experiencing a notable increase in demand for clinical trials. This surge is driven by the need for innovative therapies and the growing focus on personalized medicine. As pharmaceutical companies and research organizations seek to expedite drug development, the adoption of eclinical solutions becomes essential. In 2025, the Italian clinical trial market is projected to reach approximately €1.5 billion, reflecting a compound annual growth rate (CAGR) of around 8%. This growth indicates a robust environment for eclinical solutions, as they facilitate efficient data management and regulatory compliance, ultimately enhancing the trial process.
Government Initiatives for Digital Health
The Italian government is actively promoting digital health initiatives, which significantly impact the eclinical solution market. Policies aimed at enhancing healthcare infrastructure and integrating technology into clinical practices are being implemented. For instance, the National Recovery and Resilience Plan allocates substantial funding for digital transformation in healthcare. This initiative is expected to increase the adoption of eclinical solutions by approximately 25% over the next few years. Such government support not only fosters innovation but also encourages collaboration between public and private sectors, thereby strengthening the overall eclinical solution market.
Increased Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, research institutions, and technology providers, is becoming increasingly prevalent in the eclinical solution market. This trend is driven by the recognition that partnerships can enhance the efficiency and effectiveness of clinical trials. In Italy, collaborative efforts are expected to lead to the development of integrated eclinical solutions that streamline processes and improve data sharing. Such collaborations may result in a market growth rate of approximately 10% over the next few years, as stakeholders leverage each other's strengths to address common challenges and innovate within the eclinical solution market.
Emergence of Artificial Intelligence in Clinical Research
The integration of artificial intelligence (AI) into clinical research is poised to transform the eclinical solution market in Italy. AI technologies are being utilized to analyze vast amounts of data, identify patterns, and optimize trial designs. This capability not only accelerates the research process but also enhances the accuracy of outcomes. As AI adoption increases, it is anticipated that the eclinical solution market will experience a growth rate of around 15% by 2027. The potential for AI to improve patient recruitment and retention strategies further underscores its significance in shaping the future of the eclinical solution market.
















Leave a Comment