-
Report Prologue
-
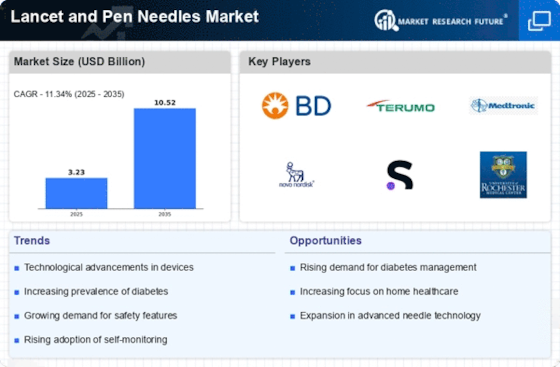
Increasing Awareness Of Lancet And Pen Needles: 15
-
Production Of Lancet And Pen Needles: 15
-
FDA Regulation For Lancet: 16
-
Market Introduction
-
Introduction 19
-
Scope Of Study 19
-
Research Objective 19
-
Assumptions & Limitations 20
- Assumptions 20
- Limitations 20
-
Market Structure 21
-
Research Methodology
-
Research Process 22
-
Primary Research 23
-
Secondary Research 23
-
Market Size Estimation 23
-
Market Dynamics
-
Introduction 25
-
Drivers 26
- Increasing Prevalence Of Diabetes 26
- Growing Incidence Number Of Contagious And Non-Contagious Diseases 27
- Increasing Inclination Towards Home Healthcare 28
- Improving Medical Device Regulation 29
-
Restraints 30
- Risk Associated With Blood Transfusion 30
- Growing Adoption Of Oral Drug Delivery Systems, Reuse Of Pen Needles And Infusion Sets 31
-
Opportunities 32
-
Mega Trends: 32
-
Macroeconomic Indicators 33
-
Market Factor Analysis
-
Porter’s Five Forces Model 34
- Bargaining Power Of Suppliers 35
- Bargaining Power Of Buyers 35
- Threat Of New Entrants 35
- Threat Of Substitutes 35
- Intensity Of Rivalry 35
-
Value Chain Analysis 36
- R&D 36
- Manufacturing 36
- Distribution & Sales 37
- Post-Sales Monitoring 37
-
Demand & Supply: Gap Analysis 37
-
Pricing Analysis 37
-
Investment Opportunity Analysis 38
-
Lancet Pen Needles Market, By Type
-
Lancet 40
- Regular Lancet 40
- Safety Lancet 40
-
Pen Needles 42
- Standard Needles 42
- Safety Needles 43
-
Lancet Pen Needles Market, By Application
-
Insulin 45
-
Capillary Blood Sampling 46
-
Hormones 47
-
Glucose Like Peptide (GLP) 47
-
Skin Testing 48
-
Lancet Pen Needles Market, By End User
-
Hospitals & Clinics 50
-
Diagnostic Centers & Medical Institutions 51
-
Home Care & Home Diagnostics 52
-
Research & Academic Laboratories 53
-
-
Lancet Pen Needles Market, By Region
-
Introduction 54
-
Europe 56
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Western Europe 59
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Germany 61
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
France 64
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
U.K 66
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Italy 69
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
SPAIN 71
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Rest Of Western Europe 74
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Eastern Europe 76
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Americas 80
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
North America 83
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
U.S. 85
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Canada 88
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
South America 90
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Asia Pacific 94
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Japan 97
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
China 99
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
India 102
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
South Korea 104
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Australia 107
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Rest Of Asia Pacific 109
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
The Middle East & Africa 113
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
UAE 116
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Saudi Arabia 118
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Kuwait 121
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Qatar 123
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Africa 126
-
Lancet Pen Needles Market, By Type
-
Lancet Pen Needles Market, By Application
-
Lancet Pen Needles Market, By End User
-
Competitive Landscape
-
Introduction 129
-
Company Share Analysis 129
-
Company Profile
-
B. Braun Melsungen AG 131
- Company Overview 131
- Financials 131
- Products 131
- Strategy 131
- Key Developments 131
-
-
Terumo Corporation 132
- Company Overview 132
- Financials 132
- Products 132
- Strategy 132
- Key Developments 132
-
Becton Dickinson 133
- Company Overview 133
- Financials 133
- Products 133
- Strategy 133
- Key Developments 133
-
Sanofi 134
- Company Overview 134
- Financials 134
- Products 134
- Strategy 134
- Key Developments 134
-
F. Hoffmann-La Roche AG 135
- Company Overview 135
- Financials 135
- Products 135
- Strategy 135
- Key Developments 135
-
Bayer AG 136
- Company Overview 136
- Financials 136
- Products 136
- Strategy 136
- Key Developments 136
-
Novo Nordisk A S 137
- Company Overview 137
- Financials 137
- Products 137
- Strategy 137
- Key Developments 137
-
Eli Lilly And Company 138
- Company Overview 138
- Financials 138
- Products 138
- Strategy 138
- Key Developments 138
-
-
Owen Mumford Ltd 139
- Company Overview 139
- Financials 139
- Products 139
- Strategy 139
- Key Developments 139
-
Medtronic 140
- Company Overview 140
- Financials 140
- Products 140
- Strategy 140
- Key Developments 140
-
Ypsomed Holding AG 141
- Company Overview 141
- Financials 141
- Products 141
- Strategy 141
- Key Developments 141
-
Sarstedt AG & Co 142
- Company Overview 142
- Financials 142
- Products 142
- Strategy 142
- Key Developments 142
-
Greiner Bio One 143
- Company Overview 143
- Financials 143
- Products 143
- Strategy 143
- Key Developments 143
-
Abbott Laboratories 144
- Company Overview 144
- Financials 144
- Products 144
- Strategy 144
- Key Developments 144
-
HTL-STREFA S.A. 145
- Company Overview 145
- Financials 145
- Products 145
- Strategy 145
- Key Developments 145
-
-
Improve Medical 146
- Company Overview 146
- Financials 146
- Products 146
- Strategy 146
- Key Developments 146
-
MRFR Conclusion
-
Key Findings 147
- From CEO’s View Point 147
- Unmet Needs Of The Market 147
-
Key Companies To Watch 148
-
Prediction Of Lancet And Pen Needles Industry 148
-
Appendix
-
Discussion Blue Print 149
-
List Of Tables
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 39
-
Lancet Pen Needles Market, BY LANCET, 2022-2030 (USD MILLION) 40
-
Lancet Pen Needles Market, BY PEN NEEDLES, 2022-2030 (USD MILLION) 42
-
Lancet Pen Needles Market, BY APPLICATION, 2022-2030 (USD MILLION) 44
-
Lancet Pen Needles Market, BY INSULIN, 2022-2030 (USD MILLION) 46
-
Lancet Pen Needles Market, BY CAPILLARY BLOOD SAMPLING, 2022-2030 (USD MILLION) 46
-
Lancet Pen Needles Market, BY HORMONES, 2022-2030 (USD MILLION) 47
-
Lancet Pen Needles Market, BY GLUCOSE LIKE PEPTIDE (GLP), 2022-2030 (USD MILLION) 47
-
Lancet Pen Needles Market, BY SKIN TESTING, 2022-2030 (USD MILLION) 48
-
Lancet Pen Needles Market, BY END USER, 2022-2030 (USD MILLION) 49
-
GLOBAL LANCET AND PEN NEEDLES MARKET BY HOSPITALS & CLINICS, 2022-2030 (USD MILLION) 51
-
GLOBAL LANCET AND PEN NEEDLES MARKET BY DIAGNOSTIC CENTERS & MEDICAL INSTITUTIONS,
-
GLOBAL LANCET AND PEN NEEDLES MARKET BY HOME CARE & HOME DIAGNOSTICS, 2022-2030
-
(USD MILLION) 52
-
GLOBAL LANCET AND PEN NEEDLES MARKET BY RESEARCH & ACADEMIC LABORATORIES,
-
Lancet Pen Needles Market, BY COUNTRY, 2022- 2030 (USD MILLION) 56
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 56
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 57
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 57
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 57
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 58
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 58
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 59
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 59
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 59
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 60
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 60
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 61
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 61
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 62
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 62
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 62
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 63
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 63
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 64
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 64
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 64
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 65
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 65
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 66
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 66
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 67
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 67
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 67
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 68
-
U.Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 68
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 69
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 69
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 69
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 70
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 70
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 71
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 71
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 72
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 72
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 72
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 73
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 73
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 74
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 74
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 74
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 75
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 75
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 76
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 76
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 77
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 77
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 77
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 78
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 78
-
Lancet Pen Needles Market, BY REGION, 2022- 2030 (USD MILLION) 80
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 80
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 80
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 81
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 81
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 82
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 82
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 83
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 83
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 83
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 84
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 84
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 85
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 85
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 86
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 86
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 86
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 87
-
U.S. Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 87
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 88
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 88
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 88
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 89
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 89
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 90
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 90
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 91
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 91
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 91
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 92
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 92
-
Lancet Pen Needles Market, BY COUNTRY, 2022- 2030 (USD MILLION) 94
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 94
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 95
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 95
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 95
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 96
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 96
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 97
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 97
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 97
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 98
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 98
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 99
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 99
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 100
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 100
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 100
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 101
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 101
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 102
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 102
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 102
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 103
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 103
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 104
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 104
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 105
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 105
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 105
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 106
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 106
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 107
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 107
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 107
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 108
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 108
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 109
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 109
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 110
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 110
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 110
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 111
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 111
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY COUNTRY, 2022- 2030 (USD MILLION) 113
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 113
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 114
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 114
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 114
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 115
-
THE MIDDLE EAST & Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 115
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 116
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 116
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 116
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 117
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 117
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 118
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 118
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 119
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 119
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 119
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 120
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 120
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 121
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 121
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 121
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 122
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 122
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 123
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 123
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 124
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 124
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 124
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 125
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 125
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 126
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 126
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 126
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 127
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 127
-
Lancet Pen Needles Market, BY TYPE, 2022-2030 (USD MILLION) 128
-
List Of Figures
-
Lancet Pen Needles Market, BY TYPE, 2022 (%) 16
-
Lancet Pen Needles Market, BY APPLICATION, 2022 (%) 17
-
Lancet Pen Needles Market, BY END USER, 2022 (%) 17
-
Lancet Pen Needles Market, BY REGION, 2022 (%) 18
-
GLOBAL LANCET AND PEN NEEDLES MARKET: MARKET STRUCTURE 21
-
RESEARCH PROCESS 22
-
PORTERS FIVE FORCES MODEL: GLOBAL LANCET AND PEN NEEDLES MARKET 34
-
Lancet Pen Needles Market, BY END USER, 2022 & 2030 (USD MILLION) 39
-
Lancet Pen Needles Market, BY APPLICATION, 2022 & 2030 (USD MILLION) 45
-
Lancet Pen Needles Market, BY END USER, 2022 & 2030 (USD MILLION) 50
-
GLOBAL LANCET AND PEN NEEDLES MARKET: COMPANY SHARE ANALYSIS, 2022 (%) 130


















Leave a Comment