-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
Introduction
-
Primary Research
-
Secondary Research
-
Market Size Estimation
-
Drivers
-
Restraints
-
Opportunities
-
Challenges
-
Macroeconomic Indicators
-
Technology Trends & Assessment
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Value Chain Analysis
-
Investment Feasibility Analysis
-
Pricing Analysis
-
Chapter 6. Global Mitral Valve Stenosis Market, by Diagnostic Test
-
Introduction
-
Electrocardiogram (ECG)
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Transthoracic Echocardiogram
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Transesophageal Echocardiogram
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Cardiac Catheterization
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Others
-
Chapter 7. Global Mitral Valve Stenosis Market, by Treatment
-
Introduction
-
Medications
- Diuretics
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Blood Thinners
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Beta Blockers
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Anti-Arrhythmic Drugs
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Antibiotics
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Procedures
- Mitral Valvuloplasty
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Mitral Valve Surgery
-
Mitral Valve Repair
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Mitral Valve Replacement
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Chapter 8. Global Mitral Valve Stenosis Market, by End User
-
Introduction
-
Hospitals & Clinics
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Diagnostic Centers
-
Market Estimates & Forecast, by Region, 2023-2032
-
Market Estimates & Forecast, by Country, 2023-2032
-
Others
-
Chapter 9. Global Mitral Valve Stenosis Market, by Region
-
Introduction
-
Americas
- North America
- South America
-
Europe
- Western Europe
- Eastern Europe
-
Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
-
Middle East & Africa
- Middle East
- Africa
-
Chapter 10. Company Landscape
-
Introduction
-
Market Share Analysis
-
Key Development & Strategies
-
Chapter 11. Company Profiles
-
Edward Lifesciences
- Company Overview
- Product Overview
- Financials Overview
- Key Developments
- SWOT Analysis
-
Boston Scientific Corporation
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
Abbott Laboratories
- Company Overview
- Product Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
HighLife Medical
- Company Overview
- Product Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Medtronic Plc
- Company Overview
- Product Overview
- Financial overview
- Key Developments
- SWOT Analysis
-
Neovasc, Inc.
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
CryoLife, Inc.
- Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
On-X Life Technologies, Inc.
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Micro Interventional Devices, Inc.
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Braun Melsungen AG
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
JenaValve Technology
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Siemens Healthcare
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Others
-
Chapter 12 MRFR Conclusion
-
Key Findings
- From CEO’s Viewpoint
- Unmet Needs of the Market
-
Key Companies to Watch
-
Predictions for the Mitral Valve Stenosis Industry
-
Chapter 13. Appendix
-
LIST OF TABLES
-
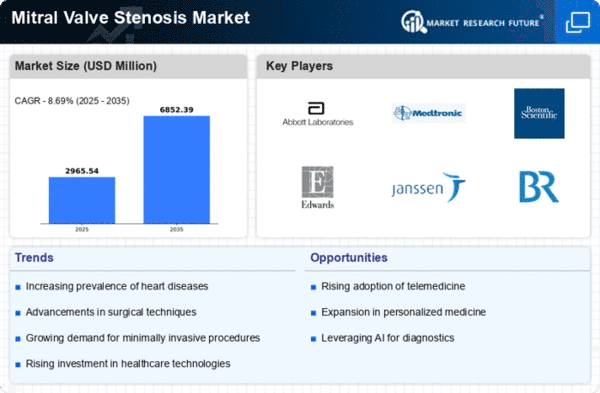
Global Mitral Valve Stenosis Market Synopsis, 2023-2032
-
Global Mitral Valve Stenosis Market Estimates and Forecast, 2023-2032
-
(USD Million)
-
Global Mitral Valve Stenosis Market, by Region, 2023-2032 (USD Million)
-
Global Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD Million)
-
Global Mitral Valve Stenosis Market, by Material, 2023-2032 (USD Million)
-
North America: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD
-
Million)
-
North America: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD
-
Million)
-
North America: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD
-
Million)
-
US: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD Million)
-
US: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD Million)
-
US: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD Million)
-
Canada: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD Million)
-
Canada: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD Million)
-
Canada: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD
-
Million)
-
South America: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD
-
Million)
-
South America: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD
-
Million)
-
South America: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD
-
Million)
-
Europe: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD Million)
-
Europe: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD Million)
-
Europe: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD Million)
-
Western Europe: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032
-
(USD Million)
-
Western Europe: Mitral Valve Stenosis Market, by Treatment, 2023-2032
-
(USD Million)
-
Western Europe: Mitral Valve Stenosis Market, by End User, 2023-2032
-
(USD Million)
-
Eastern Europe: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032
-
(USD Million)
-
Eastern Europe: Mitral Valve Stenosis Market, by Treatment, 2023-2032
-
(USD Million)
-
Eastern Europe: Mitral Valve Stenosis Market, by End User, 2023-2032
-
(USD Million)
-
Asia-Pacific: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032 (USD
-
Million)
-
Asia-Pacific: Mitral Valve Stenosis Market, by Treatment, 2023-2032 (USD
-
Million)
-
Asia-Pacific: Mitral Valve Stenosis Market, by End User, 2023-2032 (USD
-
Million)
-
Middle East & Africa: Mitral Valve Stenosis Market, by Diagnostic Test, 2023-2032
-
(USD Million)
-
Middle East & Africa: Mitral Valve Stenosis Market, by Treatment, 2023-2032
-
(USD Million)
-
Middle East & Africa: Mitral Valve Stenosis Market, by End User, 2023-2032
-
(USD Million)
-
LIST OF FIGURES
-
Research Process
-
Segmentation for Global Mitral Valve Stenosis Market
-
Segmentation Market Dynamics for Global Mitral Valve Stenosis Market
-
Global Mitral Valve Stenosis Market Share, by Diagnostic Test, 2023
-
Global Mitral Valve Stenosis Market Share, by Treatment, 2023
-
Global Mitral Valve Stenosis Market Share, by End User, 2023
-
Global Mitral Valve Stenosis Market Share, by Region, 2023
-
North America: Mitral Valve Stenosis Market Share, by Country, 2023
-
Europe: Mitral Valve Stenosis Market Share, by Country, 2023
-
Asia-Pacific: Mitral Valve Stenosis Market Share, by Country, 2023
-
Middle East & Africa: Mitral Valve Stenosis Market Share, by Country, 2023
-
Global Mitral Valve Stenosis Market: Company Share Analysis, 2023 (%)
-
Edward Lifesciences: Key Financials
-
Edward Lifesciences: Segmental Revenue
-
Edward Lifesciences: Geographical Revenue
-
Boston Scientific Corporation: Key Financials
-
Boston Scientific Corporation: Segmental Revenue
-
Boston Scientific Corporation: Geographical Revenue
-
Abbott Laboratories: Key Financials
-
Abbott Laboratories: Segmental Revenue
-
Abbott Laboratories: Geographical Revenue
-
HighLife Medical: Key Financials
-
HighLife Medical: Segmental Revenue
-
HighLife Medical: Geographical Revenue
-
Medtronic Plc: Key Financials
-
Medtronic Plc: Segmental Revenue
-
Medtronic Plc. Geographical Revenue
-
Neovasc, Inc.: Key Financials
-
Neovasc, Inc.: Segmental Revenue
-
Neovasc, Inc.: Geographical Revenue
-
CryoLife, Inc.: Key Financials
-
CryoLife, Inc.: Segmental Revenue
-
CryoLife, Inc.: Geographical Revenue
-
On-X Life Technologies, Inc.: Key Financials
-
On-X Life Technologies, Inc.: Segmental Revenue
-
On-X Life Technologies, Inc.: Geographical Revenue
-
Micro Interventional Devices, Inc.: Key Financials
-
Micro Interventional Devices, Inc.: Segmental Revenue
-
Micro Interventional Devices, Inc.: Geographical Revenue
-
Braun Melsungen AG: Key Financials
-
Braun Melsungen AG: Segmental Revenue
-
Braun Melsungen AG: Geographical Revenue
-
JenaValve Technology: Key Financials
-
JenaValve Technology: Segmental Revenue
-
JenaValve Technology: Geographical Revenue
-
Siemens Healthcare: Key Financials
-
Siemens Healthcare: Segmental Revenue
-
Siemens Healthcare: Geographical Revenue















Leave a Comment