Regulatory Support and Approvals
Regulatory support and timely approvals for new therapies are essential drivers of the Multiple Myeloma Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited review processes for innovative treatments, particularly those addressing serious conditions like multiple myeloma. The implementation of programs such as Breakthrough Therapy Designation and Fast Track Designation has facilitated quicker access to promising therapies for patients. Recent approvals of novel agents have demonstrated the effectiveness of these regulatory pathways, leading to a more dynamic market environment. This supportive regulatory framework encourages pharmaceutical companies to invest in the development of new treatments, knowing that there is a pathway for expedited approval. As a result, the regulatory landscape is likely to continue fostering innovation and enhancing the availability of effective therapies in the Multiple Myeloma Treatment Market.
Increasing Awareness and Education
The rising awareness and education surrounding multiple myeloma are pivotal in driving the Multiple Myeloma Treatment Market. Initiatives aimed at educating healthcare professionals and patients about the disease, its symptoms, and treatment options are gaining momentum. Organizations and advocacy groups are actively promoting awareness campaigns, which have led to earlier diagnosis and treatment initiation. This heightened awareness is crucial, as it encourages patients to seek medical attention promptly, ultimately improving prognosis. Furthermore, educational programs for healthcare providers are enhancing their understanding of the latest treatment modalities and clinical guidelines, enabling them to make informed decisions. As awareness continues to grow, it is expected to positively impact the demand for multiple myeloma therapies, thereby contributing to the expansion of the Multiple Myeloma Treatment Market.
Advancements in Treatment Modalities
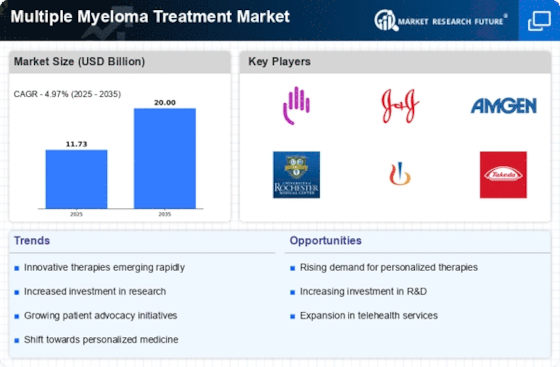
Technological advancements in treatment modalities are significantly influencing the Multiple Myeloma Treatment Market. The introduction of novel therapies, such as CAR T-cell therapy and monoclonal antibodies, has revolutionized treatment approaches, offering new hope to patients. These innovative treatments have shown promising results in clinical trials, leading to improved survival rates and quality of life for patients. For instance, the approval of therapies like daratumumab and elotuzumab has expanded the therapeutic arsenal available to clinicians. Furthermore, the integration of precision medicine into treatment protocols allows for tailored therapies based on individual patient profiles, enhancing treatment efficacy. As these advancements continue to emerge, they are expected to drive market growth by attracting investments and fostering collaborations among pharmaceutical companies, research institutions, and healthcare providers in the Multiple Myeloma Treatment Market.
Rising Incidence of Multiple Myeloma
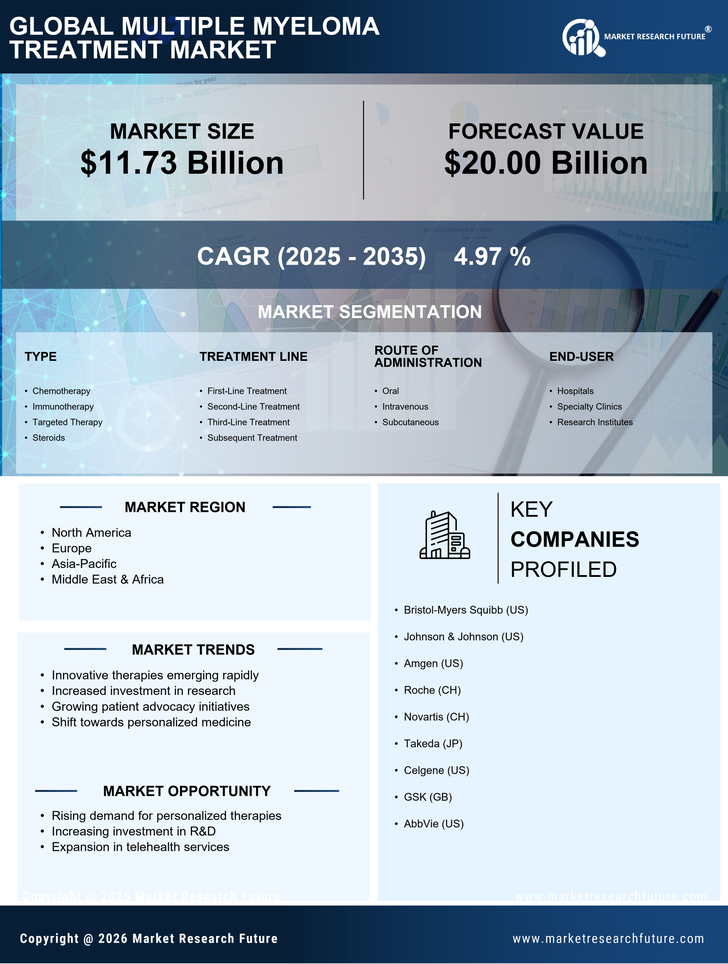
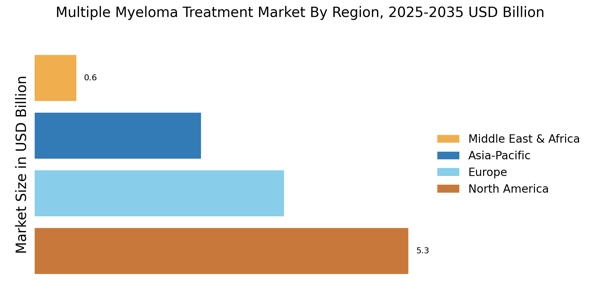
The increasing incidence of multiple myeloma is a primary driver for the Multiple Myeloma Treatment Market. Recent statistics indicate that the prevalence of this hematological malignancy is on the rise, with an estimated 32,000 new cases diagnosed annually in certain regions. This surge in cases necessitates the development and availability of effective treatment options, thereby propelling market growth. As the population ages, the risk of developing multiple myeloma increases, further contributing to the demand for innovative therapies. The growing awareness among healthcare professionals and patients about the disease also plays a crucial role in early diagnosis and treatment initiation, which is vital for improving patient outcomes. Consequently, the rising incidence of multiple myeloma is likely to stimulate investments in research and development, enhancing the overall landscape of the Multiple Myeloma Treatment Market.
Growing Investment in Research and Development
The increasing investment in research and development (R&D) is a crucial driver for the Multiple Myeloma Treatment Market. Pharmaceutical companies are allocating substantial resources to discover and develop new therapies, aiming to address unmet medical needs in multiple myeloma treatment. In recent years, R&D spending has surged, with estimates suggesting that it could reach over $10 billion annually for hematological malignancies, including multiple myeloma. This influx of funding is facilitating the exploration of novel drug candidates, combination therapies, and innovative treatment strategies. Additionally, partnerships between academia and industry are fostering collaborative research efforts, leading to accelerated drug development timelines. As a result, the focus on R&D is likely to yield breakthroughs that enhance treatment options and improve patient outcomes, thereby propelling the growth of the Multiple Myeloma Treatment Market.