-
EXECUTIVE SUMMARY
-
Market Overview
-
Key Findings
-
Market Segmentation
-
Competitive Landscape
-
Challenges and Opportunities
-
Future Outlook
-
MARKET INTRODUCTION
-
Definition
-
Scope of the study
- Research Objective
- Assumption
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
-
Forecasting Model
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Value chain Analysis
-
Porter's Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Regional Impact
- Opportunity and Threat Analysis
-
New-Born Screening Market, BY TEST TYPE (USD BILLION)
-
Disorders Screening
-
Genetic Screening
-
Hearing Screening
-
Congenital Heart Disease Screening
-
New-Born Screening Market, BY TECHNOLOGY (USD BILLION)
-
Mass Spectrometry
-
Liquid Chromatography
-
Immunoassays
-
DNA Sequencing
-
New-Born Screening Market, BY END USER (USD BILLION)
-
Hospitals
-
Diagnostic Laboratories
-
Research Institutions
-
New-Born Screening Market, BY REGION (USD BILLION)
-
North America
-
Europe
-
Asia Pacific
-
Latin America
-
Middle East and Africa
-
New-Born Screening Market, BY REGIONAL (USD BILLION)
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
-
APAC
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Indonesia
- Rest of APAC
-
South America
- Brazil
- Mexico
- Argentina
- Rest of South America
-
MEA
- GCC Countries
- South Africa
- Rest of MEA
-
COMPETITIVE LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market share Analysis
-
Major Growth Strategy in the Newborn Screening Market
-
Competitive Benchmarking
-
Leading Players in Terms of Number of Developments in the Newborn Screening Market
-
Key developments and growth strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales and Operating Income
- Major Players R&D Expenditure. 2023
-
COMPANY PROFILES
-
Neogen Corporation
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Abbott Laboratories
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Genoox
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
BD
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Thermo Fisher Scientific
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Ambry Genetics
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
LifeLabs
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
GeneDx
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
LabCorp
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Roche Diagnostics
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Sonic Healthcare
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Natus Medical
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
PerkinElmer
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Masimo
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Invitae
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
LIST OF TABLES
-
LIST OF ASSUMPTIONS
-
NORTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
US NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
US NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
US NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
US NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
US NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CANADA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
CANADA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
CANADA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CANADA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
CANADA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GERMANY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
GERMANY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
GERMANY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GERMANY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
GERMANY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
UK NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
UK NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
UK NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
UK NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
UK NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
FRANCE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
FRANCE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
FRANCE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
FRANCE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
FRANCE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
RUSSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
RUSSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
RUSSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
RUSSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
RUSSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ITALY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
ITALY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
ITALY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ITALY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
ITALY NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SPAIN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SPAIN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SPAIN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SPAIN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
SPAIN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CHINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
CHINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
CHINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CHINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
CHINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
INDIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
INDIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
INDIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
JAPAN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
JAPAN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
JAPAN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
JAPAN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
JAPAN NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MALAYSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MALAYSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MALAYSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MALAYSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
MALAYSIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
THAILAND NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
THAILAND NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
THAILAND NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
THAILAND NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
THAILAND NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDONESIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
INDONESIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
INDONESIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDONESIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
INDONESIA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
REST OF APAC NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
BRAZIL NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
BRAZIL NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
BRAZIL NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
BRAZIL NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
BRAZIL NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEXICO NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MEXICO NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MEXICO NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEXICO NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
MEXICO NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ARGENTINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
ARGENTINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
ARGENTINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ARGENTINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
ARGENTINA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGION, 2019-2035 (USD BILLIONS)
-
REST OF MEA NEWBORN SCREENING MARKET SIZE ESTIMATES & New-Born Screening Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
PRODUCT LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
-
ACQUISITION/PARTNERSHIP
-
LIST OF FIGURES
-
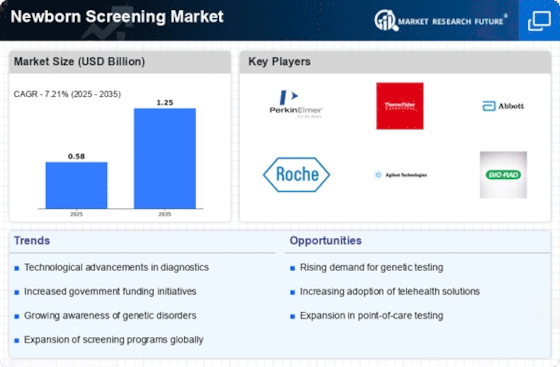
MARKET SYNOPSIS
-
NORTH AMERICA NEWBORN SCREENING MARKET ANALYSIS
-
US NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
US NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
US NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
US NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
US NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
CANADA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
CANADA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
CANADA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
CANADA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
CANADA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
EUROPE NEWBORN SCREENING MARKET ANALYSIS
-
GERMANY NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
GERMANY NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
GERMANY NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
GERMANY NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
GERMANY NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
UK NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
UK NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
UK NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
UK NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
UK NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
FRANCE NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
FRANCE NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
FRANCE NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
FRANCE NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
FRANCE NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
RUSSIA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
RUSSIA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
RUSSIA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
RUSSIA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
RUSSIA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
ITALY NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
ITALY NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
ITALY NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
ITALY NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
ITALY NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
SPAIN NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
SPAIN NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
SPAIN NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
SPAIN NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
SPAIN NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
REST OF EUROPE NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
REST OF EUROPE NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
REST OF EUROPE NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
REST OF EUROPE NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
REST OF EUROPE NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
APAC NEWBORN SCREENING MARKET ANALYSIS
-
CHINA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
CHINA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
CHINA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
CHINA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
CHINA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
INDIA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
INDIA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
INDIA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
INDIA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
INDIA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
JAPAN NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
JAPAN NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
JAPAN NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
JAPAN NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
JAPAN NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
SOUTH KOREA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
SOUTH KOREA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
SOUTH KOREA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
SOUTH KOREA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
SOUTH KOREA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
MALAYSIA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
MALAYSIA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
MALAYSIA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
MALAYSIA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
MALAYSIA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
THAILAND NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
THAILAND NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
THAILAND NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
THAILAND NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
THAILAND NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
INDONESIA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
INDONESIA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
INDONESIA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
INDONESIA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
INDONESIA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
REST OF APAC NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
REST OF APAC NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
REST OF APAC NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
REST OF APAC NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
REST OF APAC NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS
-
BRAZIL NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
BRAZIL NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
BRAZIL NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
BRAZIL NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
BRAZIL NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
MEXICO NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
MEXICO NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
MEXICO NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
MEXICO NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
MEXICO NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
ARGENTINA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
ARGENTINA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
ARGENTINA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
ARGENTINA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
ARGENTINA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
REST OF SOUTH AMERICA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
MEA NEWBORN SCREENING MARKET ANALYSIS
-
GCC COUNTRIES NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
GCC COUNTRIES NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
GCC COUNTRIES NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
GCC COUNTRIES NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
GCC COUNTRIES NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
SOUTH AFRICA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
SOUTH AFRICA NEWBORN SCREENING MARKET ANALYSIS BY TECHNOLOGY
-
SOUTH AFRICA NEWBORN SCREENING MARKET ANALYSIS BY END USER
-
SOUTH AFRICA NEWBORN SCREENING MARKET ANALYSIS BY REGION
-
SOUTH AFRICA NEWBORN SCREENING MARKET ANALYSIS BY REGIONAL
-
REST OF MEA NEWBORN SCREENING MARKET ANALYSIS BY TEST TYPE
-
REST OF MEA NEWBORN SCREENING MARKET ANALYSIS

















Leave a Comment