-
EXECUTIVE SUMMARY
-
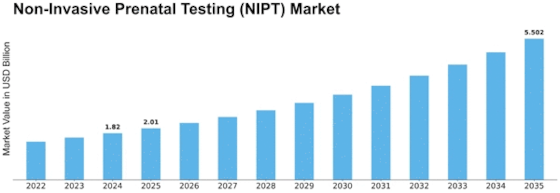
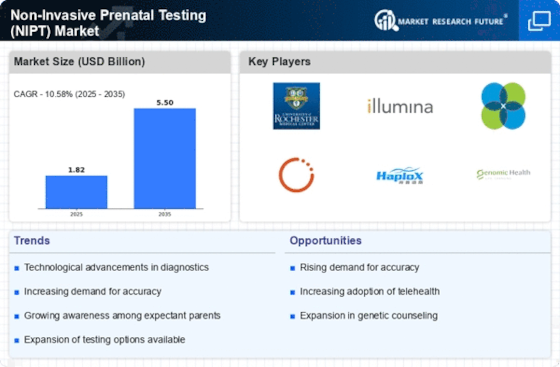
Market Overview
-
Key Findings
-
Market Segmentation
-
Competitive Landscape
-
Challenges and Opportunities
-
Future Outlook
-
MARKET INTRODUCTION
-
Definition
-
Scope of the study
- Research Objective
- Assumption
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
- Primary Interviews and Information Gathering Process
- Breakdown of Primary Respondents
-
Forecasting Model
-
Market Size Estimation
- Bottom-Up Approach
- Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Value chain Analysis
-
Porter's Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
- Regional Impact
- Opportunity and Threat Analysis
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TEST TYPE (USD BILLION)
-
Cell-Free Fetal DNA Testing
-
Biochemical Markers Testing
-
Ultrasound Screening
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY APPLICATION (USD BILLION)
-
Trisomy Detection
-
Single Gene Disorders
-
Sex Chromosome Abnormalities
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TECHNOLOGY (USD BILLION)
-
Next-Generation Sequencing
-
Microarray Analysis
-
Massively Parallel Sequencing
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY END USER (USD BILLION)
-
Hospitals
-
Diagnostic Laboratories
-
Research Institutions
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY REGIONAL (USD BILLION)
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
-
APAC
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Indonesia
- Rest of APAC
-
South America
- Brazil
- Mexico
- Argentina
- Rest of South America
-
MEA
- GCC Countries
- South Africa
- Rest of MEA
-
COMPETITIVE LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market share Analysis
-
Major Growth Strategy in the Non-Invasive Prenatal Testing (NIPT) Market
-
Competitive Benchmarking
-
Leading Players in Terms of Number of Developments in the Non-Invasive Prenatal Testing (NIPT) Market
-
Key developments and growth strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales and Operating Income
- Major Players R&D Expenditure. 2023
-
COMPANY PROFILES
-
Natera
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Sequenom
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Maternita
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Center for Disease Control and Prevention
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Quest Diagnostics
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Roche
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Illumina
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
LabCorp
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Verinata Health
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
GENEASIS
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Harmony Prenatal Test
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Fetal Medicine Foundation
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Eurofins Scientific
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Mayo Clinic
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
LIST OF TABLES
-
LIST OF ASSUMPTIONS
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019-2035 (USD BILLIONS)
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY APPLICATION, 2019-2035 (USD BILLIONS)
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019-2035 (USD BILLIONS)
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY END USER, 2019-2035 (USD BILLIONS)
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET SIZE ESTIMATES & Non Invasive Prenatal Testing Market, BY REGIONAL, 2019-2035 (USD BILLIONS)
-
PRODUCT LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
-
ACQUISITION/PARTNERSHIP
-
LIST OF FIGURES
-
MARKET SYNOPSIS
-
NORTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
US NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
CANADA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
GERMANY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
UK NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
FRANCE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
RUSSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
ITALY NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
SPAIN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
REST OF EUROPE NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
CHINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
INDIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
JAPAN NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
SOUTH KOREA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
MALAYSIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
THAILAND NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
INDONESIA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
REST OF APAC NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
BRAZIL NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
MEXICO NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
ARGENTINA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
REST OF SOUTH AMERICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
GCC COUNTRIES NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
SOUTH AFRICA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TEST TYPE
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY APPLICATION
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY TECHNOLOGY
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY END USER
-
REST OF MEA NON-INVASIVE PRENATAL TESTING (NIPT) MARKET ANALYSIS BY REGIONAL
-
KEY BUYING CRITERIA OF NON-INVASIVE PRENATAL TESTING (NIPT) MARKET
-
RESEARCH PROCESS OF MRFR
-
DRO ANALYSIS OF NON-INVASIVE PRENATAL TESTING (NIPT) MARKET
-
DRIVERS IMPACT ANALYSIS: NON-INVASIVE PRENATAL TESTING (NIPT) MARKET
-
RESTRAINTS IMPACT ANALYSIS: NON-INVASIVE PRENATAL TESTING (NIPT) MARKET
-
SUPPLY / VALUE CHAIN: NON-INVASIVE PRENATAL TESTING (NIPT) MARKET
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TEST TYPE, 2025 (% SHARE)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TEST TYPE, 2019 TO 2035 (USD Billions)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY APPLICATION, 2025 (% SHARE)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY APPLICATION, 2019 TO 2035 (USD Billions)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2025 (% SHARE)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY TECHNOLOGY, 2019 TO 2035 (USD Billions)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY END USER, 2025 (% SHARE)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY END USER, 2019 TO 2035 (USD Billions)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY REGIONAL, 2025 (% SHARE)
-
NON-INVASIVE PRENATAL TESTING (NIPT) Non Invasive Prenatal Testing Market, BY REGIONAL, 2019 TO 2035 (USD Billions)
-
BENCHMARKING OF MAJOR COMPETITORS
-
"

















Leave a Comment