Advancements in Gene Therapy
Innovations in gene therapy are emerging as a transformative force within the Ornithine Transcarbamylase OTC Deficiency Treatment Market. Recent developments in genetic engineering techniques, such as CRISPR and viral vector delivery systems, hold promise for providing long-term solutions for patients with this deficiency. These advancements could potentially lead to curative therapies, significantly altering the treatment landscape. As research progresses, the market may witness a shift towards more personalized medicine approaches, enhancing treatment efficacy and patient outcomes. The anticipated approval of gene therapies in the coming years could catalyze growth in the market, attracting investment and interest from pharmaceutical companies.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy organizations is playing a crucial role in shaping the Ornithine Transcarbamylase OTC Deficiency Treatment Market. These groups are instrumental in raising awareness about the condition, promoting research funding, and advocating for better treatment options. Their efforts contribute to increased visibility of the disorder, which can lead to enhanced diagnosis rates and improved access to therapies. Furthermore, these organizations often collaborate with healthcare providers and pharmaceutical companies to facilitate clinical trials and patient education initiatives. As the influence of these advocacy groups continues to grow, they are likely to drive demand for effective treatments and support services for individuals affected by Ornithine Transcarbamylase OTC deficiency.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development of innovative therapies within the Ornithine Transcarbamylase OTC Deficiency Treatment Market. Initiatives aimed at expediting the approval process for new treatments, particularly for rare diseases, are becoming more prevalent. This regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that their products may receive faster market access. Additionally, the establishment of clear guidelines for clinical trials and post-marketing surveillance enhances the overall safety and efficacy of new therapies. As a result, the market is likely to experience a wave of new treatment options that could significantly improve the quality of life for patients with Ornithine Transcarbamylase OTC deficiency.
Increased Investment in Rare Disease Research
The Ornithine Transcarbamylase OTC Deficiency Treatment Market is benefiting from heightened investment in research focused on rare diseases. Pharmaceutical companies and research institutions are increasingly allocating resources to develop therapies for conditions that have historically been overlooked. This trend is partly driven by regulatory incentives, such as orphan drug designations, which provide financial benefits and market exclusivity for developers. As a result, the market is likely to see a surge in clinical trials and new product launches aimed at addressing the unmet needs of patients with Ornithine Transcarbamylase OTC deficiency. This influx of investment may lead to innovative treatment options and improved patient care.
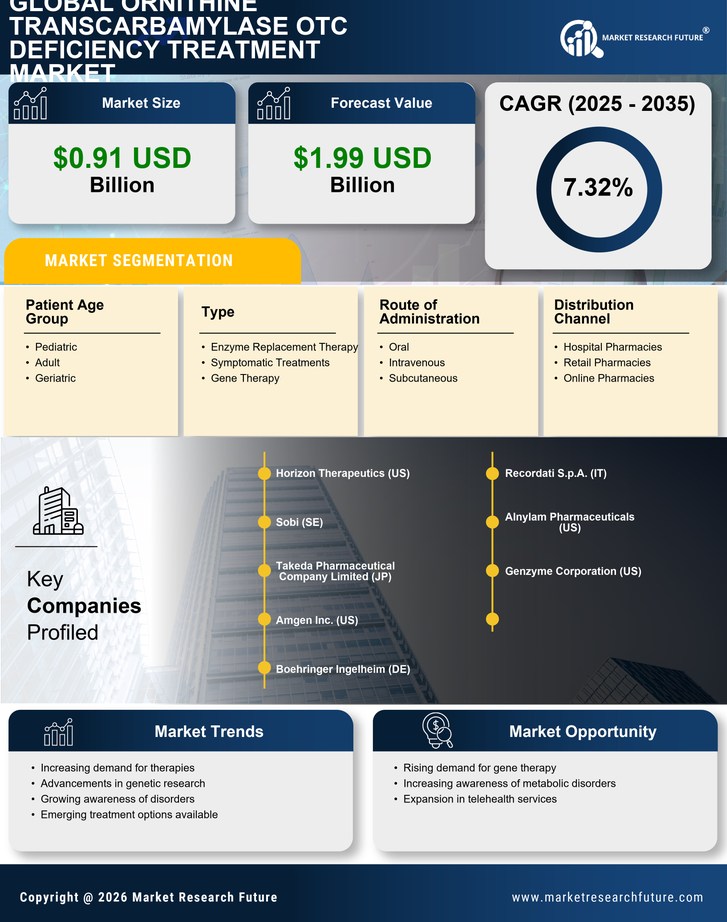
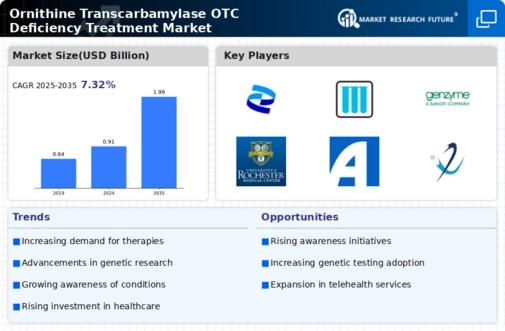
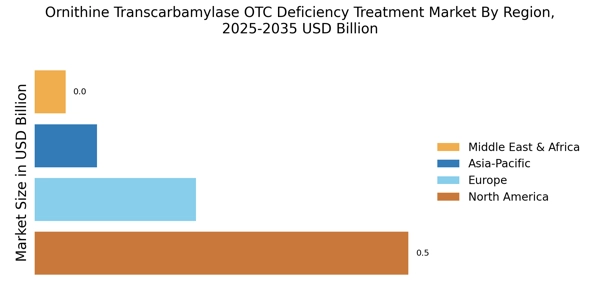
Rising Prevalence of Ornithine Transcarbamylase OTC Deficiency
The increasing incidence of Ornithine Transcarbamylase OTC deficiency is a pivotal driver for the Ornithine Transcarbamylase OTC Deficiency Treatment Market. Recent estimates suggest that this genetic disorder affects approximately 1 in 80,000 births, leading to a growing patient population requiring effective treatment options. As awareness of metabolic disorders rises, more individuals are being diagnosed, which in turn fuels demand for specialized therapies. The need for effective management strategies for patients, particularly in pediatric populations, is becoming increasingly critical. This trend indicates a potential expansion in the market as healthcare providers seek to address the needs of affected individuals, thereby driving innovation and investment in treatment solutions.