· Sterile Drugs
· Medical Devices
· Biologics
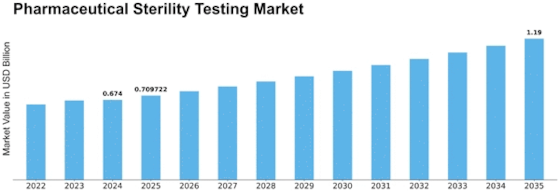
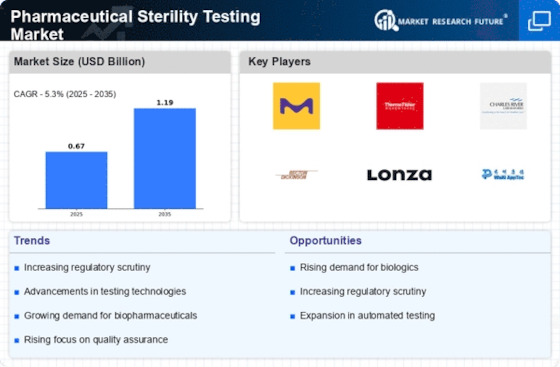
Pharmaceutical Sterility Testing Product Type Format Outlook (USD Billion, 2018-2032)
· Instruments
· Kits & Reagents
· Services
Pharmaceutical Sterility Testing Type Outlook (USD Billion, 2018-2032)
· In-house
· Outsourcing
· Sterility Testing
· Bioburden Testing
· Bacterial Endotoxin Testing
· Compounding Pharmacies
· Medical Devices Companies
· Pharmaceutical Companies
· North America Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o US Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o CANADA Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
· Europe Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Germany Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o France Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o UK Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o ITALY Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o SPAIN Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Rest Of Europe Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
· Asia-Pacific Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-house
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o China Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Japan Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o India Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Australia Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Rest of Asia-Pacific Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
· Rest of the World Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Middle East Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Africa Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies
o Latin America Outlook (USD Billion, 2018-2032)
o Pharmaceutical Sterility Testing Market by Sample
§ Sterile Drugs
§ Medical Devices
§ Biologics
o Pharmaceutical Sterility Testing Market by Product Type Format
§ Instruments
§ Kits & Reagents
§ Services
o Pharmaceutical Sterility Testing Market by Type
§ In-House
§ Outsourcing
o Pharmaceutical Sterility Testing Market by Test Type
§ Sterility Testing
§ Bioburden Testing
§ Bacterial Endotoxin Testing
o Pharmaceutical Sterility Testing Market by End User
§ Compounding Pharmacies
§ Medical Devices Companies
§ Pharmaceutical Companies

















Leave a Comment