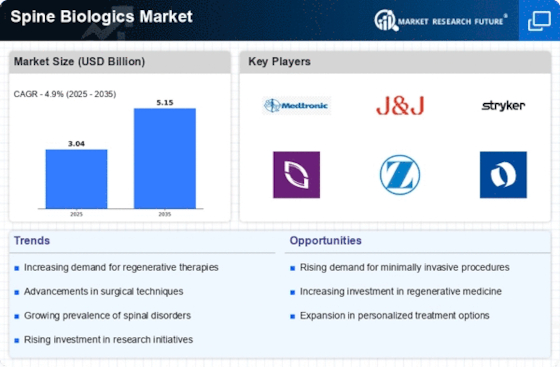
Top Industry Leaders in the Spine Biologics Market

Latest Spine Biologics Companies Update:
Medtronic (US) Acquired Mazo BioSciences, Inc. (US) in August 2023, strengthening its biologics portfolio with Mazo's Osteocel Plus® bone graft substitute, offering enhanced fusion rates and patient outcomes.
Orthofix International N.V. (Belgium) Launched its Trinity System in June 2023, a combined interbody spacer and bone graft substitute for spinal fusion surgeries, aiming to simplify surgical procedures and improve bone fusion rates.
Stryker (US) Collaborated with TissueGenesis, Inc. (US) to distribute TissueGenesis' osteochondral allograft cartilage repair solutions for spinal applications, expanding treatment options for patients with cartilage damage.
Regenerative Biologics, Inc. (US) Announced the positive reasults of a Phase II clinical trial for its Osteocel-HA bone graft substitute for spinal fusion, demonstrating improved fusion rates compared to traditional bone graft options
MiMedX Group, Inc. (US) Received FDA approval for its Puregraft® Flowable Micro DBM putty for spinal fusion procedures in July 2023, offering a moldable bone graft option for surgeons for improved surgical site adaptation.
List of Spine Biologics Key companies in the market
- Arthrex, Inc.
- Cesca Therapeutics Inc.
- DePuy Synthes
- Orthopaedic Seminar
- Exactech, Inc.
- K2M, Inc.
- Lattice Biologics Ltd
- Medtronic
- NuTech Spine, Inc.
- NuVasive Inc.
- Orthofix Holdings, Inc.
- Regen Lab USA LLC
- RTI Surgical Holdings, Inc.
- Stryker
- Wright Medical Group N.V.
- XTANT MEDICAL
- Zimmer Biomet
- Smith & Nephew
- Dr PRP USA LLC