

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Market Size Snapshot
| Year | Value |
|---|---|
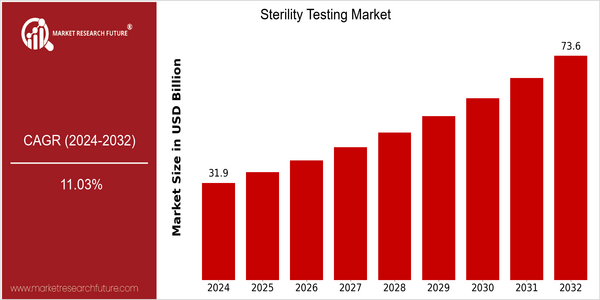
| 2024 | USD 31.88 Billion |
| 2032 | USD 73.62 Billion |
| CAGR (2024-2032) | 11.03 % |
Note – Market size depicts the revenue generated over the financial year
The global sterility testing market is poised to grow at a significant CAGR from 2024 to 2032, from a current value of $31.88 billion to $ 73.62 billion. This remarkable growth is reflected in a CAGR of 11.03% for the forecast period. The growing demand for sterile products in the pharmaceutical, biotechnological, and medical device industries is mainly responsible for this growth. The regulatory authorities are still implementing strict quality control measures to ensure the safety and efficacy of the products. Further driving the market are the technological developments in the development of rapid sterility tests and automation of laboratory processes. In this respect, the leading companies in the field are Merck KGaA, Charles River Laboratories, and Thermo Fisher Scientific. Strategic alliances and acquisitions are also contributing to the sterility testing market.
Regional Market Size
Regional Deep Dive
The sterility testing market is growing at a fast pace across the globe, driven by advancements in testing technology, regulatory requirements and a greater focus on patient safety. In North America, the stringent regulations of the Food and Drug Administration and the growing pharmaceutical industry are driving the sterility testing market. Europe is characterized by a strong focus on quality assurance and compliance. The Asia-Pacific region is characterized by a high growth potential due to increasing investments in the healthcare industry and a growing biopharmaceutical industry. The Middle East and Africa are gradually adopting sterility testing practices, influenced by rising healthcare standards and regulatory frameworks. Latin America is also gaining importance in the sterility testing market as the pharmaceutical and biotechnology industries are becoming more aware of the need for sterility testing.
Europe
- The European Medicines Agency (EMA) has introduced new regulations that require enhanced sterility testing for biologics, pushing companies to adopt more advanced testing methodologies.
- Innovations in rapid sterility testing technologies, such as the use of molecular methods, are gaining traction among European manufacturers, leading to faster turnaround times and improved compliance.
Asia Pacific
- Countries like China and India are investing heavily in their biopharmaceutical sectors, leading to an increased demand for sterility testing services and products.
- The rise of contract research organizations (CROs) in the region is facilitating access to advanced sterility testing solutions, enabling local companies to meet international quality standards.
Latin America
- The Latin American market is witnessing a surge in demand for sterility testing due to increasing investments in the pharmaceutical and biotechnology sectors, particularly in Brazil and Mexico.
- Regulatory bodies in the region are starting to align more closely with international standards, which is driving the need for compliance in sterility testing practices.
North America
- The FDA has recently updated its guidelines on sterility testing, emphasizing the need for more rigorous testing protocols, which is expected to drive innovation and investment in testing technologies.
- Key players like Merck and Charles River Laboratories are expanding their capabilities in sterility testing, focusing on automation and rapid testing methods to meet the increasing demand from the pharmaceutical industry.
Middle East And Africa
- The World Health Organization (WHO) is promoting better healthcare practices in the region, which includes the adoption of sterility testing in pharmaceutical manufacturing.
- Local governments are beginning to implement stricter regulations regarding sterility testing, which is expected to enhance the overall quality of healthcare products in the region.
Did You Know?
“Did you know that sterility testing is not only crucial for pharmaceuticals but also for medical devices, with the latter being subject to stringent testing protocols to ensure patient safety?” — International Organization for Standardization (ISO)
Segmental Market Size
The Sterility Testing Market is a critical part of the pharmaceutical and biotech industries. It is currently growing due to the stricter regulatory environment and the increased awareness of product safety. The major drivers of the market are the regulatory requirements of the FDA and the EMA, which require sterility testing for medical devices and pharmaceuticals. In addition, the rapid sterility testing methods are driving the market. The sterility testing market is currently in the process of being adopted. The leading companies in the adoption of sterility testing are Merck and Charles River Laboratories. The main application is the testing of injectable drugs, surgical instruments and biologicals, where sterility is critical. The emergence of the swine flu pandemic has increased the importance of sterility testing and increased the level of investment in sterility testing. In addition, the development of sterility testing systems and real-time monitoring tools has accelerated the process of sterility assurance.
Future Outlook
The sterility testing market is poised for significant growth from 2024 to 2032, with a projected CAGR of 11.03% from the period of 2024 to 2032. This growth is mainly driven by the growing demand for sterile products in the pharmaceutical, biotechnology and medical device industries, where stringent regulatory requirements have necessitated strict sterility testing procedures. As the world's population ages and the prevalence of chronic diseases increases, the need for safe and effective medical solutions will increase, which is expected to reach around 70% penetration of sterility testing in these industries by 2032. Technological developments will play an important role in shaping the future of the sterility testing market. The introduction of rapid sterility testing methods, automation of testing processes and the use of artificial intelligence for data analysis will improve the efficiency and accuracy of the test, thereby reducing the time to market for new products. Furthermore, the ongoing emphasis on quality assurance and compliance with international standards will increase the demand for advanced sterility testing solutions. Also, the emergence of new trends such as the rise of personalized medicine and the development of biopharmaceuticals will further drive the adoption of advanced sterility testing methods, thereby ensuring the continued evolution of the market.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Market Size Value In 2023 | USD 28.29 Billion |
| Growth Rate | 11.03% (2024-2032) |
Sterility Testing Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









