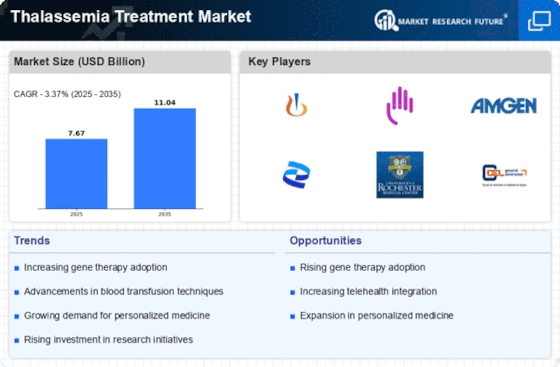
Top Industry Leaders in the Thalassemia Treatment Market
 Latest Thalassemia Treatment Companies UpdateNov 2023: The world's inaugural gene therapy treatment for sickle cell disease has been approved by Britain's drugs authority, a development that may provide thousands of individuals in the country suffering from the debilitating illness with respite. According to a release, the first medication authorized to use the CRISPR gene editing technology, Casgevy, was approved by the Medicines and Healthcare Regulatory Agency. The product's creators were awarded the 2020 Nobel Prize. The commission approved using Casgevy, a drug produced by Boston-based Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics, as a treatment for individuals 12 and older with sickle cell disease and thalassemia. Up until now, the only permanent cure available has been bone marrow transplants, which are incredibly difficult operations with highly terrible side effects. To enable the body to produce healthy hemoglobin, the novel medication Casgevy targets the faulty gene in an individual's bone marrow stem cells. Patients undergo a round of chemotherapy before using stem cells from the individual's bone marrow and genetic editing techniques in a lab to correct the gene. After that, the patient receives a permanent treatment when the cells are reinfused. At least two hospital stays are required for patients: one for collecting stem cells and another for delivering the modified cells.List of Thalassemia Treatment Key companies in the market
Latest Thalassemia Treatment Companies UpdateNov 2023: The world's inaugural gene therapy treatment for sickle cell disease has been approved by Britain's drugs authority, a development that may provide thousands of individuals in the country suffering from the debilitating illness with respite. According to a release, the first medication authorized to use the CRISPR gene editing technology, Casgevy, was approved by the Medicines and Healthcare Regulatory Agency. The product's creators were awarded the 2020 Nobel Prize. The commission approved using Casgevy, a drug produced by Boston-based Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics, as a treatment for individuals 12 and older with sickle cell disease and thalassemia. Up until now, the only permanent cure available has been bone marrow transplants, which are incredibly difficult operations with highly terrible side effects. To enable the body to produce healthy hemoglobin, the novel medication Casgevy targets the faulty gene in an individual's bone marrow stem cells. Patients undergo a round of chemotherapy before using stem cells from the individual's bone marrow and genetic editing techniques in a lab to correct the gene. After that, the patient receives a permanent treatment when the cells are reinfused. At least two hospital stays are required for patients: one for collecting stem cells and another for delivering the modified cells.List of Thalassemia Treatment Key companies in the market
- Novartis AG (Switzerland)
- Bluebird Bio, Inc. (US)
- Kiadis Pharma (Netherlands)
- CELGENE CORPORATION (US)
- Sangamo Therapeutics (US)
- Acceleron Pharma, Inc. (US)
- Gamida Cell (Israel)